AUTHOR: Stephanie Schuster, PhD, Senior Technical Support Scientist, Advanced Materials Technology 20 Years of High…

Current LC/MS Approaches For PFAS Testing with Ultrashort and Long Chain Mixtures
AUTHOR:
Conner McHale, Technical Support Specialist, Advanced Materials Technology
INTRODUCTION to PFAS ANALYTICAL METHODS
Per- and polyfluoroalkyl substances (PFAS) are a group of chemicals used to make fluoropolymer coatings and products that resist heat, oil, stains, grease, and water. These toxic “forever chemicals” are a concern to health and environment, thus increasing regulation by the Environmental Protection Agency.
Short chain PFAS such as trifluoracetic acid (TFA) and pentafluoropropionic acid (PFPrA) are challenging analytes to analyze due to low retention and poor peak shape using typical reversed phase high pressure liquid chromatography (HPLC) conditions and LC/MS/MS. Although mixed mode hydrophilic interaction liquid chromatography (HILIC) has been demonstrated to improve retention, this approach has limitations and reversed phase methods are preferred.
A new reversed phase, superficially porous particle (SPP) silica with a positive charge surface chemistry has shown advantages for short and long chain PFAS HPLC analyses compatible with several mass spectrometry platforms.
THE PFAS FAMILY:
PFAS are man-made chemicals that are found everywhere including cleaning products, water-resistant fabrics, nonstick cookware, firefighting foam, and even greaseresistant paper due to its unique chemical properties. Over the years, PFAS exposure has become a growing concern to the human health/ environment and now compounds such as PFOA and PFOS are no longer manufactured in the United States.
PFAS can be grouped into two broad categories: nonpolymeric and polymeric molecules. Non-polymeric PFAS can be further subdivided into two groups represented by perfluoroalkyl and polyfluoroalkyl substances. The formerincludes molecules wherein the hydrophobic carbon chain is totally fluorinated with the exception of the terminal end, which hosts a polar functional group such as carboxylate (COO−), sulfonate (SO3−) or phosphate (OPO3 −) which confers hydrophilicity.2 (as seen in Figure 1)
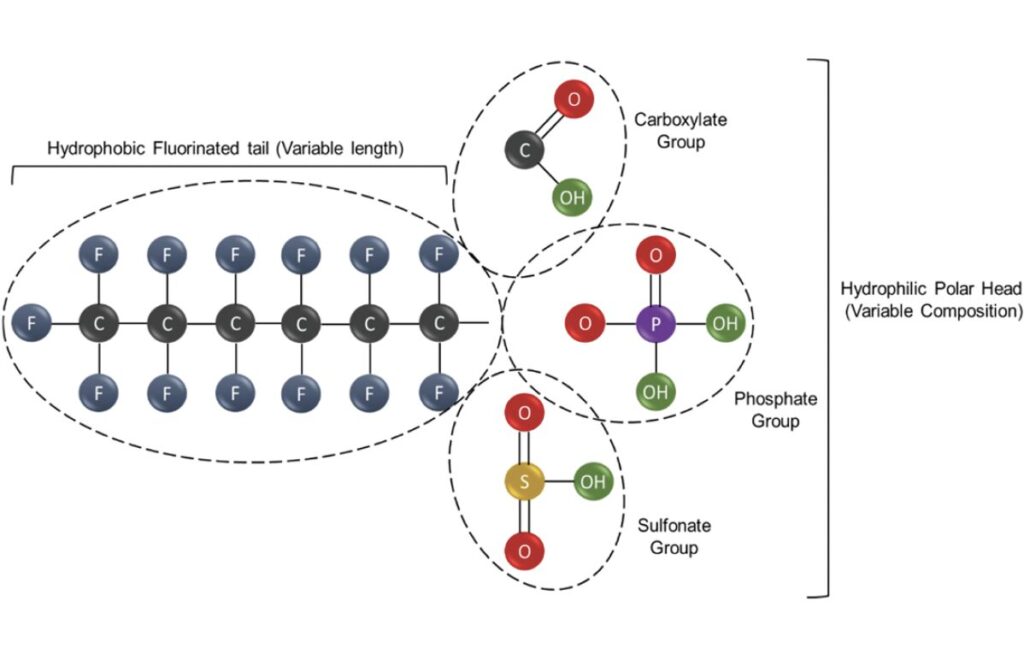
Figure 1: Typical structure of non-polymeric PFAS consisting of a hydrophobic tail and hydrophilic polar head2
In response to concern over long-chain PFASs (defined as having between 8 and 14 fluorinated carbon atoms), industries have transitioned to using short- and ultrashort-chain perfluorocarboxlyic acids (PFCAs) and perfluorosulfonic acids (PFSAs) as replacements. Short-chain PFASs have between four and seven fluorinated carbon atoms, and ultrashort-chain PFASs have fewer than four fluorinated carbon atoms.3
Thousands of PFAS variations have been discovered using LC/MS/MS and GC/MS technology. With continual improvements in instrument and column technology, more and more variations are being discovered each day.
KEY WORDS:
PFAS, EPA 537.1, EPA 8327, MS/MS, HALO® PFAS, HALO® PCS Phenyl-Hexyl, superficially porous particles, Fused-Core®
Environmental Protection Agency (EPA) Analytical PFAS Testing Methods
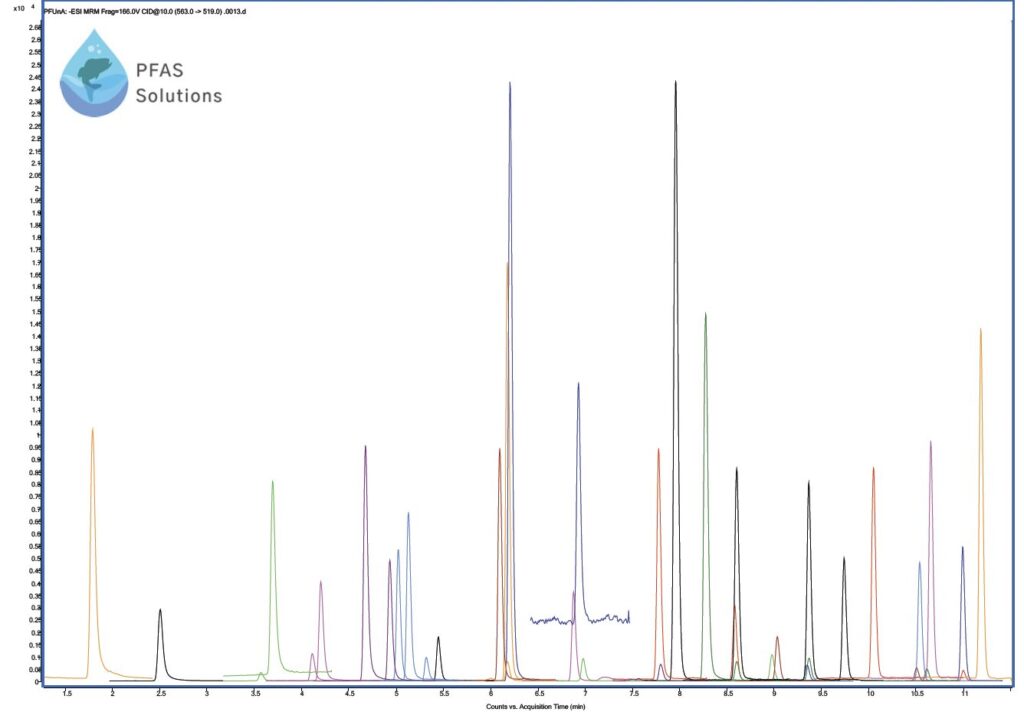
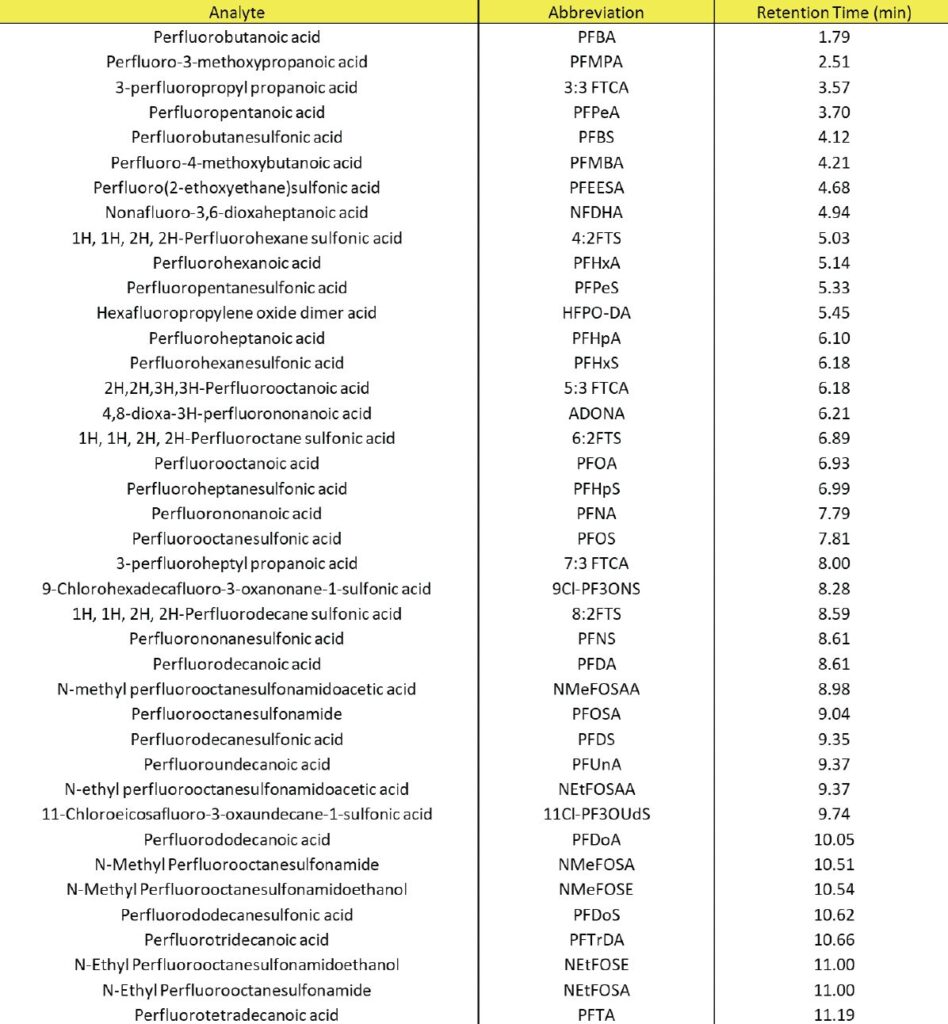
PFAS exposure through drinking water has become one of the many concerns to the public. As analytical instruments improved overtime, especially the commercial use of the electrospray interface for LC/MS/MS, methods were now able to quantitate PFAS compounds, some at concentration levels down to parts per trillion. As regulations started to come into effect, the EPA ultimately developed validated HPLC methods involving analysis of PFAS in water by LC-MS/ MS. For drinking (potable) water methods 537.1, 537, and 533 are recommended while non-potable water and other environmental media include methods 8327 and 1633. The latest EPA 1633 method includes 40 PFAS compounds across nine different compound classes (including linear and branched isomers) using a reversed phase C18 column along with an analytical delay column. For example, Figure 2 represents a fortified soil sample that has been spiked with each PFAS standard at 5 ppb following solid phase extraction.
Link to Full Report with Test Methods
Table 1: EPA 1633 Analyte List with Retention Times
Ultra-Short Chain PFAS/ The Challenge
The limited number of studies on the environmental occurrence of ultrashort-chain PFASs is primarily due to the analytical difficulties in measuring these compounds. Although techniques exist for measuring ultrashort-chain PFASs, many require additional instrumentation or a combination of multiple analytical techniques.3 One of the problems analyzing short chain PFAS is low retention under reversed phase conditions like EPA 1633. Even under high aqueous conditions retention for short chain PFAS becomes a challenge for standard C18 column chemistries. Having analytes too close to the column void can also cause issues with any unwanted interferences or ionization suppressing species, making it more difficult to accurately quantitate and measure the peak of interest.
Hydrophilic Interaction Liquid Chromatography (HILIC) can be considered when analyzing polar PFAS analytes that are not well retained by reversed phase, however, it can have its limitations in terms of full PFAS panels involving the long chain compounds. Differences in sample solvent can also be challenging while running under HILIC conditions due to the high percentage of organic modifiers required for analysis.
A new reversed phase, superficially porous particle (SPP) silica with a positive charge surface chemistry from Advanced Materials Technology has shown advantages for short chain PFAS HPLC analyses. With the addition of the charged surface ligand, retention is increased for the ultrashort PFAS compounds allowing reversed phase HPLC to be a viable option. The HALO® PCS columns incorporate a 90 Å, 2.7μm SPP particle including C18 and Phenyl-Hexyl ligand options.
Experimental PFAS Testing Method Development (C1-C18 PFAS Carbon Length)
Mixtures of PFAS are resolved using a combination of acetonitrile or methanol and water with various acidic mobile phase modifiers. A 90 Å, 2.7μm superficially porous particle with a positive charge surface on the stationary phase allows adequate retention for the short chain PFAS such as TFA, PFPrA and PFBA (PCS C18, Advanced Materials Technology). The LC separation has been systematically optimized using DryLab computational tools (Molnar-Institute) to acquire high throughput and high-resolution separations of both short chain and more complex long chain PFAS. Analytical conditions (mobile phases and flow rate) were selected for favorable operational sensitivity and selectivity for three MS systems, including the triple quadrupole (3Q, Shimadzu), QExactive HF Orbitrap (ThermoScientific) and Quadrupole Time of Flight (Q-TOF, Agilent) MS instruments.
Link to Full Report with Test Methods
Standard PFAS and isotopically labeled internal standards were obtained as mixtures from Wellington Labs and LGC. Separations were conducted with gradient elution using both methanol and acetonitrile as organic modifiers, to map retention and resolution parameters for DryLab gradient, flow rate, and ESI conditions optimization. Several buffers were used during method optimization including ammonium formate, ammonium acetate, and combinations of acidic modifiers and buffer. Overall, the combination of ammonium formate and formic acid showed best results for adequate retention and peak shape.
Comparison of typical C18 bonded-phase materials versus hybrid positively charged surface C18 materials indicated a significant useful increase in TFA, PFPrA and PFBA short chain PFAS retention, while allowing for resolution of long chain PFAS compounds. (Figure 3 ) Modest effects of acidic modifiers are noted for both retention, peak shape, and resolution. The differences in methanol and acetonitrile as organic modifiers are consistent with previous observations for solvent strength on reversed phase retention while the use of a superficially porous particle (SPP) particle permits excellent efficiencies with suitable system back pressure.
A broad gradient ranging from 2-95% organic was used in order to retain the polar and non-polar analytes. A separation of the ultra-short and long chain PFAS can be seen in figure 3, showing excellent retention for the ultrashort chain PFAS using a HALO 90 Å, 2.7μm PCS Phenyl Hexyl column with the combination of a HALO® PFAS delay column.
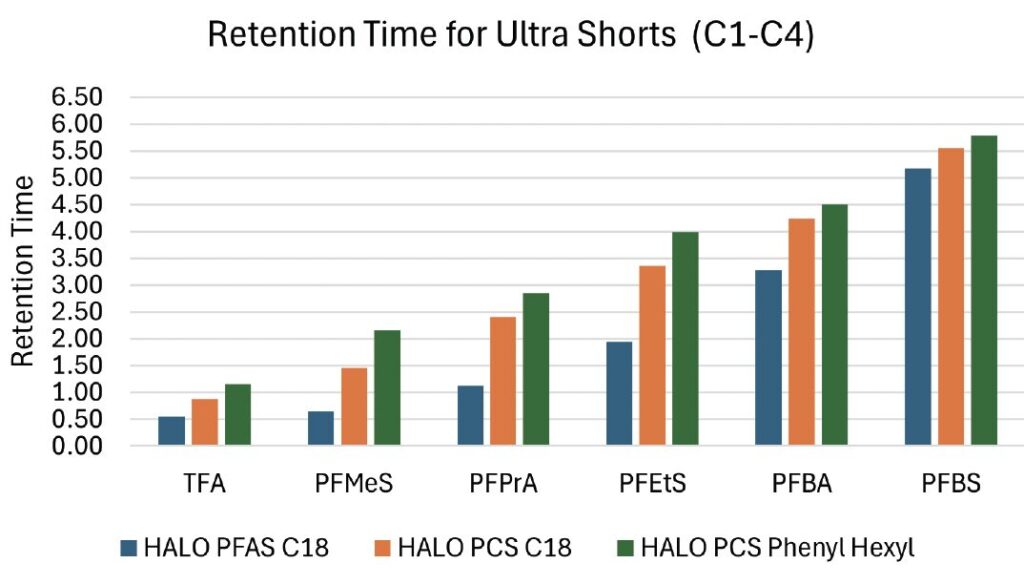
Figure 3: HALO® column retention time comparison of ultra-short chain PFAS
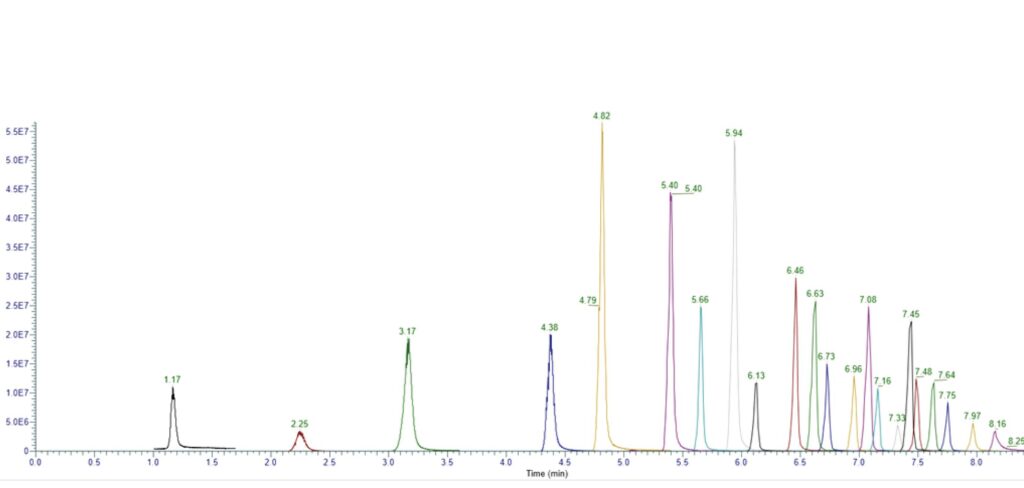
Figure 4: Analysis of Ultra-short and Long Chain PFAS using a HALO® PCS Phenyl-Hexyl Column
Peak Identities:
1. TFA (1.17, m/z: 113.2, CE: 15)
2. PFMtS (2.25, m/z: 148.95, CE: 47)
3. PFPrA (3.17, m/z: 163.3, CE: 14)
4. PFEtS (4.38, m/z: 199.25, CE: 48)
5. PFBA (4.82, m/z: 213.00, CE: 16)
6. PFPrS (5.40, m/z: 249.20, CE: 48)
7. PFPeA (5.66, m/z: 263.00, CE: 16)
8. PFBS (5.94, m/z: 299.00, CE: 48)
9. PFHxA (6.13, m/z: 313.00, CE: 24)
10. PFHpA (6.46, m/z: 363.00, CE: 18)
11. PFHxS (6.63, m/z: 399.00, CE: 46)
12. PFOA (6.73, m/z: 413.00, CE: 17)
13. PFNA (6.96, m/z: 463.00, CE: 21)
14. PFOS (7.08, m/z: 499.00, CE: 45)
15. PFDA (7.16, m/z: 513.00, CE: 17)
16. PFUnA (7.33, m/z: 563.00, CE: 18)
17. PFDS (7.45, m/z: 599.00, CE: 48)
18. PFDoA (7.48, m/z: 613.00, CE: 23)
19. PFTriA (7.64, m/z: 663.00, CE: 29
20. PFTreA (7.75, m/z: 713.00, CE: 20)
21. PFHxDA (7.97, m/z: 813.00, CE: 19)
22. PFODA (8.16, m/z: 913.00, CE: 18)
PFAS HPLC methods are continuing to improve including newer methods involving no solid phase extraction steps, reducing time and money in laboratory workflows. This is achieved by injecting a large amount of sample on column. Below is an example of an ultrashort chain PFAS analysis in a well water sample, avoiding solid phase extraction and injecting a large injection volume (20μL) for analysis.
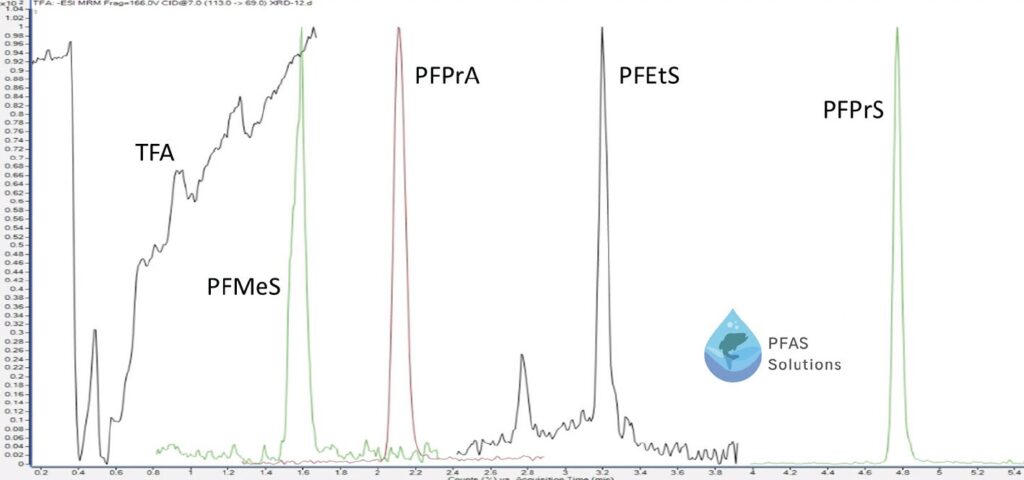
Figure 5: Ultrashort chain PFAS analysis in Well Water
CONCLUSION:
Reversed phase HPLC with the combination of LC/MS/MS is the most common approach to analyze PFAS compounds. As HPLC column technologies along with improvements to LC/MS sensitivity continue to grow, more and more of these analytes are being discovered. A reversed phase positive charge surface chemistry has proven to be effective analyzing not only the short and long chain PFAS, but ultrashort chain PFAS compounds as well.
Link to Full Report with Test Methods
Acknowledgements:
Charles R. Powley, Ph.D., Chief Scientist STRIDE Center for PFAS Solutions
References:
1. PFAS Analytical Methods Development and Sampling Research | US EPA
2. Panieri, E., Baralic, K., Djukic-Cosic, D., Buha Djordjevic, A., & Saso, L. (2022). PFAS Molecules: A Major Concern for the Human Health and the Environment. Toxics, 10(2), 44. https://doi.org/10.3390/ toxics10020044
3. Rapid and Simultaneous Quantification of Short- and Ultrashort-Chain Perfluoroalkyl Substances in Water and Wastewater, Paige Jacob and Damian E. Helbling, ACS ES&T Water 2023 3 (1), 118-128,DOI:10.1021/ acsestwater.2c00446



