AUTHOR: Conner McHale, Technical Support Specialist, Advanced Materials Technology INTRODUCTION to PFAS ANALYTICAL METHODS Per-…

20 Years of High Quality Fused-Core® Column Manufacturing
AUTHOR:
Stephanie Schuster, PhD, Senior Technical Support Scientist, Advanced Materials Technology
20 Years of High Quality Fused-Core® Column Manufacturing
INTRODUCTION
Since our founding in 2005, Advanced Materials Technology has been focused on one mission – Improving the way the sample travels through the column on the way to the detector. Using our novel Fused-Core® superficially porous particles, we challenged conventional wisdom regarding particle design and engineered innovative solutions for separation scientists around the world. Our objectives are to improve separation speed, resolution, throughput and overall satisfaction for our customers through better technology and service. Equally importantly, our quality procedures and practices are integrated into every HALO® column delivered, ensuring your success – every time. Advanced Materials Technology is a certified ISO 9001:2015 company that follows an extensive Quality Management System.
THE MAKING OF THE HALO® PARTICLE
1. QUALITY BY DESIGN
Along with the innovation that is demonstrated by HALO® products, the quality of HALO® is unsurpassed, which stems from AMT’s complete control over the entire manufacturing process – from the starting high quality raw materials to the final packed column with controls and measurements built into every step. The creation of a HALO® column begins with the manufacturing of the solid silica core, which is produced using high quality, electronic-grade raw materials. As the particle’s foundation, it is essential to closely monitor the manufacture of the core. This first step of the process is characterized by more than 45 different in-process controls and 4 separate QC/QA tests. Tight tolerances are in place to guarantee that the core is made reproducibly and consistently every time. Figure 1 demonstrates how little variation there has been in the 1.7 µm core for the 2.7 µm HALO® particles over the span of the product lifetime. This result contributes to our commitment for our customers’ long-term repeatability of separations utilizing AMT powered HPLC columns.
2. CORE CONSISTENCY

Figure 1. Mean particle size of the 1.7 µm core over the span of 20 years. With an RSD of < 0.08%, the consistency of the process is evident.
3. THE SHELL
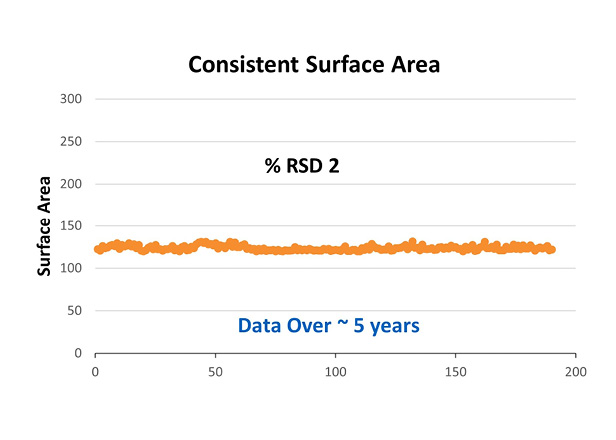
Next, the porous silica shell is added to the core by coating the core with several layers of highly pure, monosized silica nanoparticles. Here AMT has complete control over the production and quality of the nanoparticles to produce a uniform pore size and shell thickness thus leading to a consistent and reliable surface area as demonstrated in Figure 2. This consistency in surface area from lot to lot and year after year yields excellent chromatographic reproducibility. Addition of the shell to the core consists of more than 100 in-process controls and 7 rigorous QC/QA tests proving how diligently the thickness of the porous shell is controlled and monitored.
The pore size of HALO® particles is obtained by careful selection of the nanoparticles that are used in the process. The pores in the porous shell of HALO® particles are determined by the size of the nanoparticles that are used to form the porous shell. The pores are the open spaces between the nanoparticles that become bigger when the nanoparticles become bigger. The size of the analytes of interest for the separation dictates the pore size to be used for their analysis, with larger pores designed for biomolecules and smaller pores designed for small molecules. AMT has 5 different pore size products: 90 Å, 120 Å, 160 Å, 400 Å, and 1000 Å.
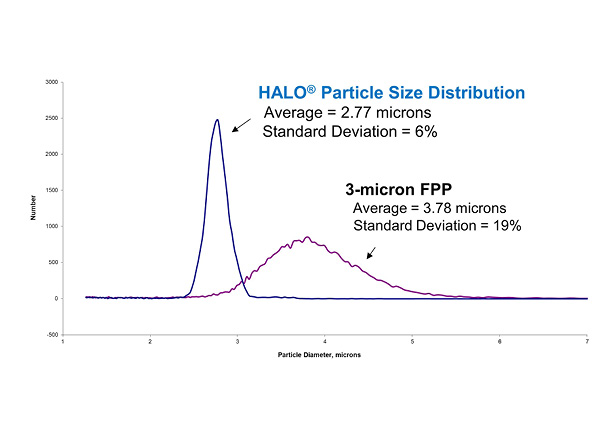
AMT silica superficially porous particle (SPP) manufacturing results in a very narrow particle size distribution that is a hallmark of HALO® column quality. This narrow particle size distribution contributes to high efficiency columns with stable chromatographic beds. An example of the particle size distribution for 2.7 µm HALO® particles compared to typical 3 µm fully porous particles is shown in Figure 3. The AMT SPP process has a standard deviation more than 3 times smaller than the fully porous particle (FPP) manufacturing process.
4. SURFACE OPTIMIZATION
Next AMT uses a proprietary method to create uniform and consistent surface silanols on the HALO® particles. Once this step is complete the particles are ready for bonding or can be used as non-derivatized or bare silica. AMT uses a challenging chromatographic test at pH 7 with a basic analyte to confirm the uniformity of the surface silanols. Figure 4 illustrates lot to lot consistency for several years’ worth of HALO® C18 batches.
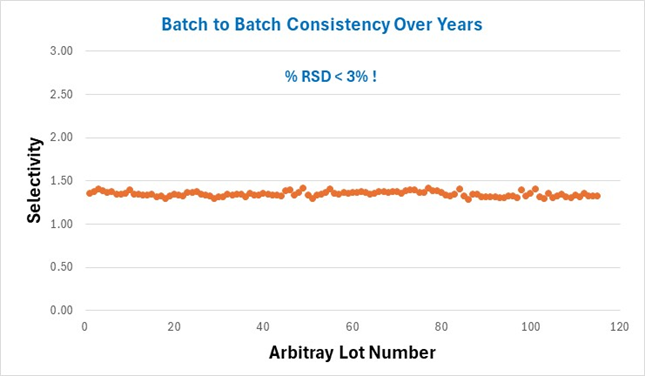
Figure 4. Selectivity between a neutral and basic compound for HALO® C18 lots over several years showing % RSD of < 3%.
5. BONDING

The finished HALO® particles are then ready for derivatization with the appropriate stationary phase for our small molecule, biomolecule and our application-specific columns. The bonding process consists of 25 in-process controls and 20 QC/QA tests. Figure 5 shows an example QA test that is used for evaluating HALO® C18. This test is run to qualify that the reaction to derivatize the surface with the stationary phase has been successfully completed as demonstrated by tight specifications for retention and selectivity.
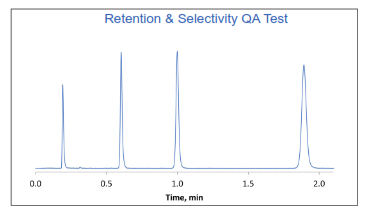
Figure 5. Example QA test that examines the retention and selectivity of 2 µm HALO® C18.
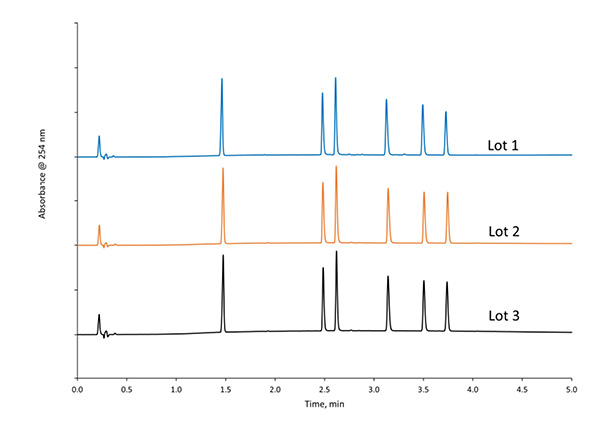
Figure 6 shows the lot-to-lot reproducibility for 3 different lots of HALO® Elevate C18, 2.7 µm, a column designed for high-pH applications. A mix of 1 neutral compound and 5 different bases was run using a gradient of pH 10.7 ammonium hydroxide and acetonitrile with 2.1 x 50 mm columns. The average %RSD across the retention times for all of the compounds was 0.3% demonstrating excellent lot-to-lot reproducibility.
Excellent lot-to-lot reproducibility is shown spanning a period of 4 years using the HALO 160 Å ES-C18 stationary phase and a sample of adalimumab tryptic digest in Figure 7. Lot performance such as this is crucial for customers developing methods that will be used today, tomorrow, and years from now.
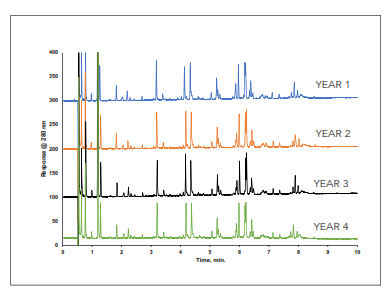
Figure 7. Excellent lot-to-lot reproducibility over the span of 4 years for an adalimumab tryptic digest run on HALO 160 Å ES-C18, 2.7 µm, 2.1 x 150 mm columns. Gradient using water/0.1% DFA and acetonitrile/0.1% DFA. 0.6 mL/min and 60 °C.
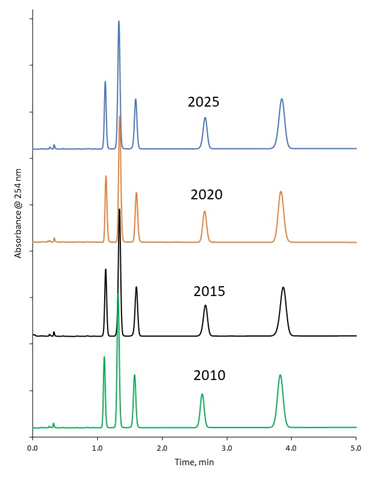
To illustrate the lot-to-lot consistency of HALO® columns over an even larger time span, columns loaded using archive lots of 2.7 µm HALO® C18 from 2010, 2015, and 2020 were tested and compared to a column loaded with a current lot of 2.7 µm HALO® C18 manufactured in 2025. Figure 8 shows the highly reproducible results for the HALO® C18 lots spanning 15 years. The average %RSD over all of the peaks for retention time is 0.8%.
6. STABILITY
Along with excellent reproducibility, HALO® columns are also known for their stability. The longevity of our superficially porous technology is showcased at low pH and high temperature for 3000 injections (approximately 23,000 column volumes); the results illustrate no significant changes as shown in Figure 9.
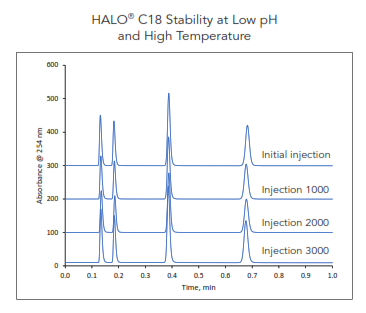
Figure 9. Long column lifetime of HALO® C18 demonstrated using 60 °C and pH 2 mobile phase conditions for 3000 injections or ~ 23,000 column volumes.
One of the newest HALO® stationary phases is Elevate C18, which is designed for operation at high pH and high temperature. The stability of Elevate C18 was tested at a challenging environment of high pH 10 and 60 °C. The column demonstrated superior performance (no loss in retention and no changes in peak shape and back pressure) for over 500 injections (>20,000 column volumes of mobile phase consumption). See Figure 10.
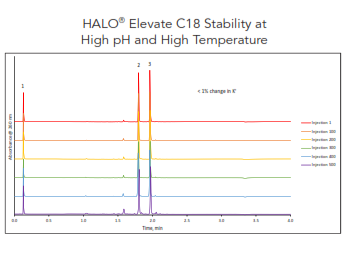
Figure 10. Example showing excellent stability for HALO® Elevate C18, 2.7 µm, 2.1 x 50 mm. Gradient using 10 mM ammonium bicarbonate and acetonitrile. 0.8 mL/min and 60 °C. Peak identities in order: uracil, acenaphthene, and amitriptyline.
7. QA/QC TESTING
After the HALO® particles are bonded and pass their qualification tests, they are ready to be packed into columns. AMT uses a proprietary automated process to pack HALO® columns to eliminate any variation in packing quality. This step includes 20 in-process controls and 6 QC/QA tests. Every column is tested and must pass tight criteria for theoretical plates, tailing factor, and selectivity before it is qualified as acceptable. See Figure 11 for more than 5 years’ worth of efficiency data for 90 Å HALO® C18, 2.7 µm, 4.6 x 150 mm columns.
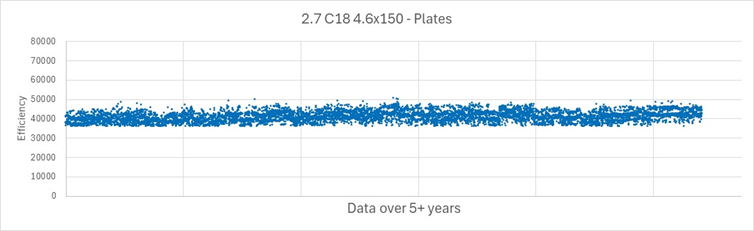
Figure 11. Exceptional efficiency for HALO® C18 columns ranging from a reduced plate height of 1.1 to 1.5.
OUR JOURNEY ENDS WITH YOU!
The final step in the process of manufacturing a column is packaging. HALO® columns are packaged with their quality assurance reports and Care and Use documentation. All packaging materials are 100% recyclable. See Figure 12 for an example of a quality assurance report.
Figure 12. Example HALO® quality assurance report.
HALO® columns are sold globally and ship quickly. To find your local distributor, please visit https://halocolumns.com/find-a-distributor/. AMT is committed to ship columns within 24 hours of receipt of an order. So whether you have been a customer from the beginning or are just learning about HALO® columns, you should have confidence that the product you receive today will be of the highest quality and consistent throughout the lifetime of the method that you develop with it.





