AUTHOR: Stephanie Schuster, PhD, Senior Technical Support Scientist, Advanced Materials Technology 20 Years of High…

Enhancing the Sensitivity of Top-Down and Bottom-Up Proteomic Applications Using the New HALO® 1.5 MM ID Column
AUTHOR: Andrew Harron Ph.D., Application Scientist
MARKET SEGMENT: BIOCLASS
ABSTRACT
Decreasing the internal diameter of a column can increase detector response, lower solvent consumption, and bring multiple benefits to the overall analysis workflow. This has fueled the development of capillary columns which provide very high performance, but require specialized instruments in order to realize that performance. Here we present the HALO® 1.5 mm ID column, which shows enhanced sensitivity and solvent savings on a conventional UHPLC system. The only modification required to realize this performance is the reduction of extra column volume, which can easily be done by the consumer.
INTRODUCTION
The introduction of smaller diameter columns in HPLC has been an ongoing evolution in the field for a number of decades. Many advances in sensitivity and solvent savings have been realized by switching from a 4.6 mm internal diameter (ID) to a 2.1 mm ID. Typically, scaling down to a lower ID column can reduce the flow rate and solvent volume needed to reach the same optimal linear velocity, without increasing run time. In addition, sensitivity gains in the detector can be realized as well, as UV and MS, are two of the most widely used detectors in LC, and both are concentration dependent. Therefore, the maximum signal, or peak height for the analyte of interest, is directly related to the maximum concentration of the analyte while it is being analyzed. Thus, when the same sample volume is injected on both a larger ID and a smaller ID column, there is a net increase of sample concentration on the smaller ID column, leading to greater response and increased peak height. In addition, in the case of electrospray LC-MS applications, higher ionization efficiency is typically found at lower flow rates, allowing for better desolvation, thus increasing ion production and transfer, and therefore ion current. The greater concentration of the sample on the column, coupled with the lower flow rate can enhance the overall sensitivity of the detector.1-3
However, there are factors that must be considered when changing to a smaller ID column for analysis, and paramount among them is band broadening due to extra column volume (ECV). ECV is comprised of the volumes of the mobile phase, excluding the column packing, that accumulates as the analyte traverses along the pathway from the injector to the detector. The efficiency of a column separation can be greatly affected by ECV and can vary from particle size and column dimension, however, in general, smaller diameter columns show more of an effect than larger diameter columns. This is problematic for analytes with low retention, often analyzed in high throughput applications. There are ways to minimize the ECV by incorporating minor adjustments to the system, for example optimizing the LC system by replacing the tubing from the column to the detector with low-volume tubing, which will help to minimize the dispersion.1-3
Upon minimization of ECV, the benefits that are offered with a smaller diameter column are an increase in signal response, and a decrease in mobile phase usage, benefits that can be extremely useful in proteomics and biobased applications, as long run times are often required, and lack of sensitivity can be a challenge. Here, we present the HALO® 1.5 mm ID column for proteomics analysis, including both top-down and bottom-up approaches, demonstrating gains in sensitivity and solvent savings compared to a 2.1 mm ID column.2-3
KEYWORDS: Top-down, Bottom-up, solvent savings, increased sensitivity, HALO 160 Å ES-C18, HALO 1000 Å Diphenyl
EXPERIMENTAL PEPTIDE MAPPING
Column: HALO 160 Å ES-C18, 2.7 µm, 1.5 x 150 mm
Part Number: 9212X-702
Column: HALO 160 Å ES-C18, 2.7 µm, 2.1 x 150 mm
Part Number: 92122-702
Mobile Phase A: Water/0.1% DFA
Mobile Phase B: Acetonitrile/0.1% DFA
Gradient: 2-50 %B in 60 min
Flow Rate: 0.2 mL/min for 1.5 mm ID 0.4 mL/min for 2.1 mm ID
Back Pressure: 310 bar (1.5 mm) 444 bar (2.1 mm)
Temperature: 60 °C
Detection: ESI +
Injection Volume: 2 µL of 1.25 mg/mL Trastuzumab Tryptic Digest
Sample Solvent: 1.5 M Guanidine HCl/0.5% Formic Acid
All solvents used were MS grade. Methanol, acetonitrile, mobile phase additives, and individual standards were obtained from MilliporeSigma (St. Louis, MO) unless specified otherwise.
TRYPSIN DIGESTION OF TRASTUZUMAB
Trastuzumab was denatured and alkylated using 50 mM Tris-HCl (pH 7.8)/1.5M Guanidine-HCl, and 2-iodoacetamide (Sigma Aldrich). Trypsin (Promega) was added in a ratio of 1:30 (w:w; Trypsin:mAb) followed by an incubation at 37 °C overnight. The reaction was quenched by 0.5% Formic Acid and analyzed by LCMS.
Samples were analyzed on a Shimadzu Nexera X2 (Shimadzu Scientific Instruments, USA). Mass spectra were acquired using a Thermo QE Orbitrap mass spectrometer (Bremen, Germany) using a heated electrospray (HESI-II) probe on the Ion Max source.
MS CONDITIONS
Voltage: 3.8 kV
Aux gas: 10 arbitrary units
Sheath gas: 35 arbitrary units
Sweep gas: 0 arbitrary units
Rf lens: 50 v
Heater temp: 225 °C
Capillary temp: 325 °C
EXPERIMENTAL REDUCED AND ALKYLATED AND INTACT ANALYSIS OF TRASTUZUMAB
Column: HALO 1000 Å Diphenyl, 2.7 µm, 1.5 x 150 mm
Part Number: 9212X-702
Column: HALO 1000 Å Diphenyl, 2.7 µm, 2.1 x 150 mm
Part Number: 92712-726
Mobile Phase A: Water/0.1% DFA
Mobile Phase B: 50% Acetonitrile/50% n-propanol/0.1% DFA
Gradient: 27-36 %B in 40 min
Flow Rate: 0.2 mL/min for 1.5 mm ID
0.4 mL/min for 2.1 mm ID
Back Pressure: 252 bar (1.5 mm)
272 bar (2.1 mm)
Temperature: 60 °C
Detection: PDA, 220 nm
Injection Volume: 3 µL of 1.0 mg/mL Reduced and Alkylated Trastuzumab
Sample Solvent: Water/0.1% TFA
Samples were analyzed on a Shimadzu Nexera X2 (Shimadzu Scientific Instruments, USA). Mass spectra were acquired using a Thermo QE Orbitrap mass spectrometer (Bremen, Germany) using a heated electrospray (HESI-II) probe on the Ion Max source.
MS CONDITIONS
Voltage: 3.8 kV
Aux gas: 10 arbitrary units
Sheath gas: 35 arbitrary units
Sweep gas: 0 arbitrary units
Rf lens: 50 v
Heater temp: 225 °C
Capillary temp: 320 °C
RESULTS-PEPTIDE MAPPING
Peptide mapping, a bottom-up technique, is typically used for confirmation of a monoclonal antibody (mAb) and to monitor post-translational modifications (PTMs), such as oxidation or deamidation. This method can provide site-specific information regarding PTMs, which is imperative to test for during production, processing, or storage. Multiple phases are available in the 1.5 mm ID, and for peptide mapping, the HALO® ES-C18 was selected for the stability and robust nature of the phase.
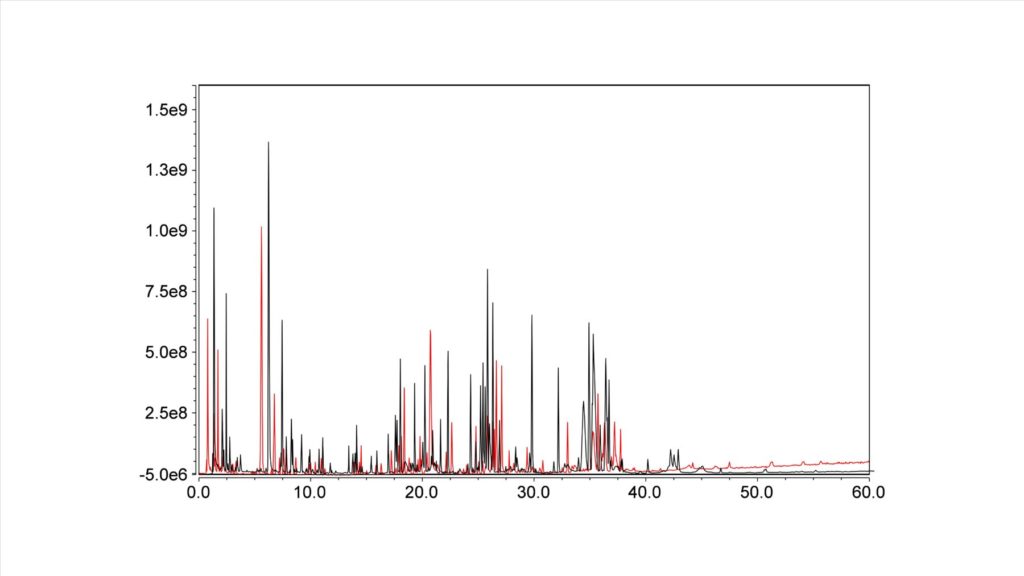
Figure 1. Trastuzumab Tryptic Digest by LCMS.
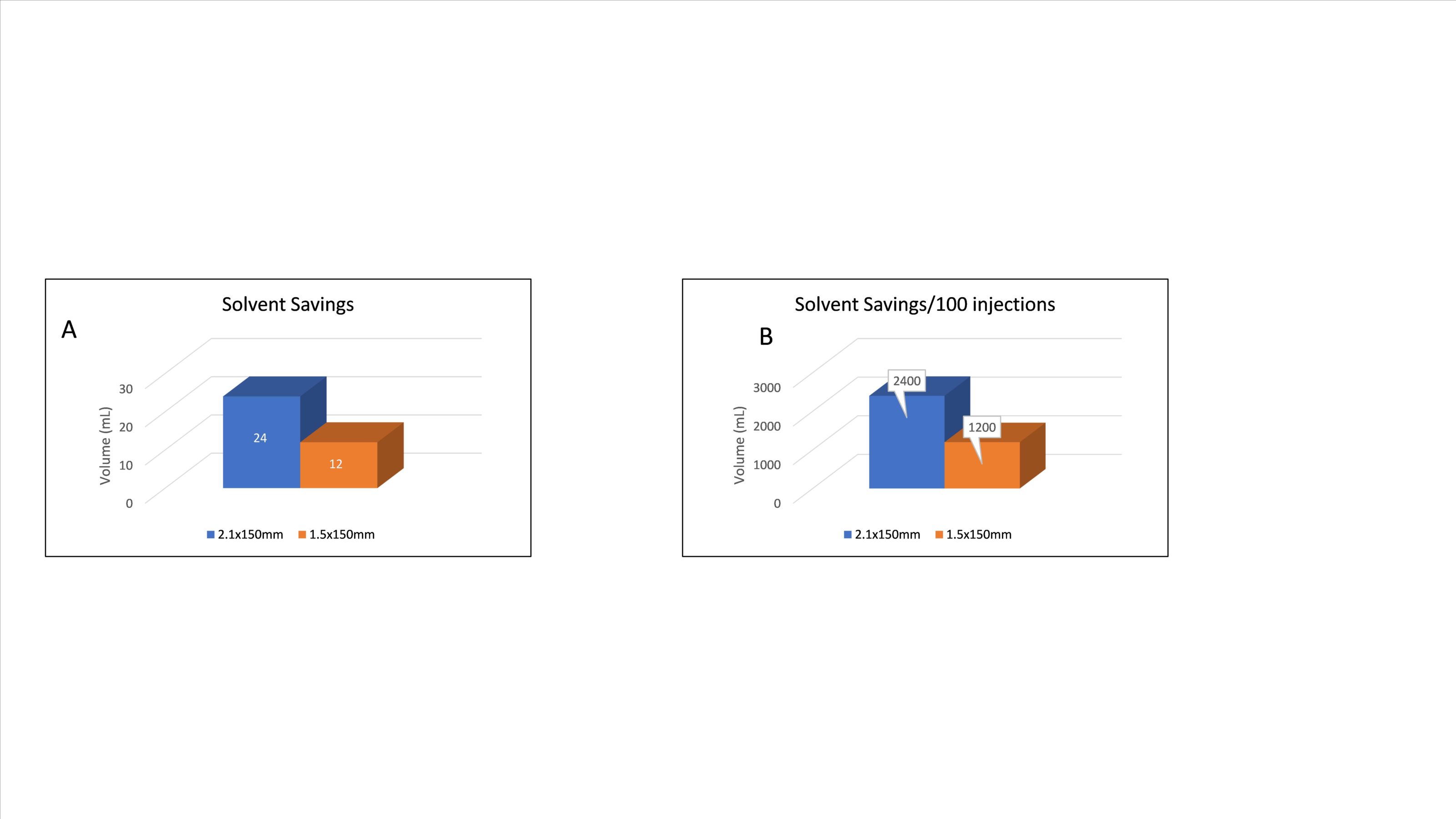
Figure 1 shows a Tryptic Digest of Trastuzumab on the HALO 160 Å ES-C18, 2.7 µm, 1.5 x 150 mm (black trace) and the HALO 160 Å ES-C18, 2.7 µm, 2.1 x 150 mm (red trace). As is clearly visible the 1.5 mm ID column has higher intensity peaks and larger areas than the 2.1 mm ID column. In addition, as this is a 60-minute run, solvent quantity becomes an issue and needs to be considered. Figure 2 shows the amount of solvent that can be saved over the course of a single run (A) and a total batch consisting of 100 injections (B), showing a savings of 2:1 for the 1.5 compared with the 2.1.
Over the course of an entire batch, the 1.5 mm ID column can not only provide higher sensitivity but also requires half the solvent compared to the 2.1 mm ID column.
Extrapolated over time, this can provide significant cost-saving measures to the consumer, as the volatility in the consumable market is very difficult to budget for. This can be especially beneficial in mAb development, for example during QA analysis and monitoring for PTMs, prior to product release.
RESULTS-REDUCED AND ALKYLATED TRASTUZUMAB
A typical workflow for bottom-up proteomics also includes the reduction of disulfide bonds to limit solvent-accessible cysteine residues. It is used for identification as it is very difficult to identify disulfide bonds with just database searching. The reduced and alkylated trastuzumab was investigated on the HALO 1000 Å Diphenyl, 2.7 µm, 1.5 x 150 and the HALO 1000 Å Diphenyl, 2.7 µm, 2.1 x 150 mm. Figure 3 shows, the light and heavy chain of the mAb separated by the Diphenyl phase. The overlayed results, of the light chain (LC) and heavy chain (HC), on the 1.5 mm (blue trace) and the 2.1 mm (red trace), clearly show the intensity gains provided by the smaller ID column, which makes the 1.5 mm an ideal choice for alkylation experiments, and overall bottom-up approaches.
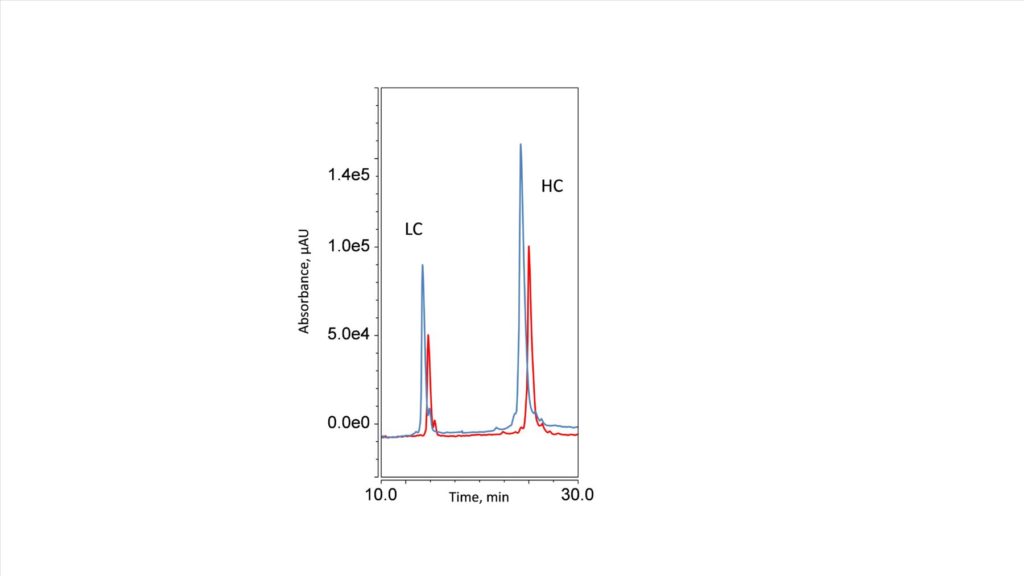
Figure 3. UV of reduced and alkylated trastuzumab showing the light chain (LC) and heavy chain (HC) on a 1.5 mm ID column (Blue) and a 2.1 mm ID column (red).
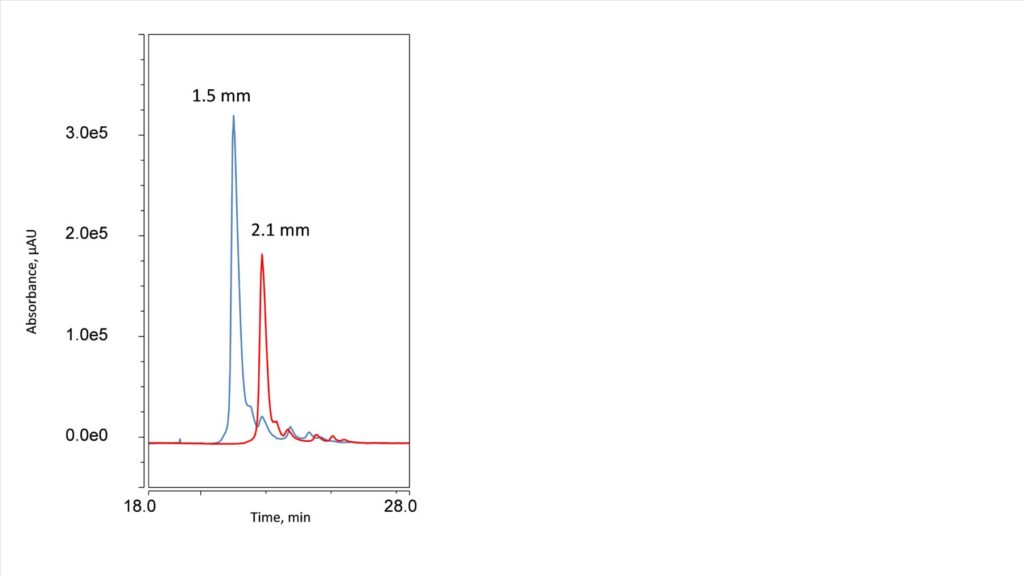
Figure 4. UV of Intact trastuzumab on a 1.5 mm ID column (Blue) and a 2.1 mm ID column (red).
RESULTS-INTACT TRASTUZUMAB
Intact mass analysis, or top-down analysis, is the determination of a protein’s total molecular weight without digestion or fragmentation and is especially useful for matching amino acid sequences and physicochemical analysis.4 The intact analysis of Trastuzumab was investigated on the HALO 1000 Å Diphenyl, 2.7 µm, 1.5 x 150 mm and the HALO 1000 Å Diphenyl, 2.7 µm, 2.1 x 150 mm. Figure 4 shows the pattern of higher sensitivity and solvent savings continued on the intact protein. The 1.5 mm ID column (blue trace) provides a 3x increase in area and peak height compared to the 2.1 mm ID column (above).
This trend is also seen in Figure 5, which shows the LCMS of the intact protein, the reduced and alkylated protein exposing the light and heavy chain, and the digested protein. The 1.5 mm column (black trace) shows an increase of 3x in ionization efficiency, compared with the 2.1 mm column (Blue trace).
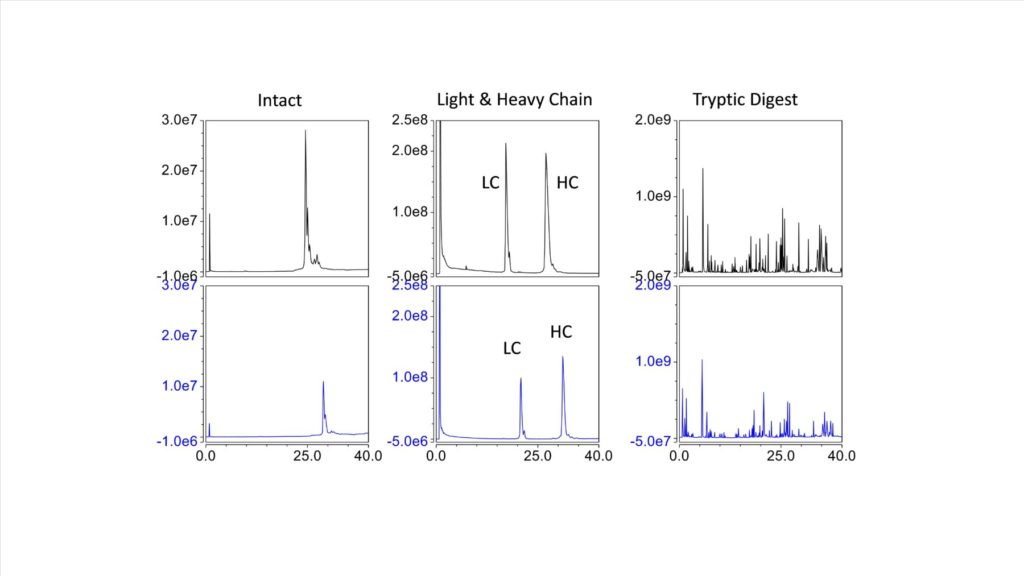
Figure 5. LCMS of the intact protein, the reduced and alkylated protein exposing the light and heavy chain, and the digested protein. The 1.5 mm column (black trace) and 2.1 mm column (Blue trace)
CONCLUSION:
Trastuzumab was analyzed in a top-down and bottom-up approach using both the HALO® Diphenyl and ES-C18 phase incorporating the new 1.5 mm ID column and compared to the 2.1 mm ID column. In all cases, the 1.5 mm ID column showed enhanced sensitivity compared to the 2.1 mm ID and provided increased solvent savings to the customer. These results, combined with the characteristic HALO® quality, reliability, and robustness, make the 1.5 mm ID column a welcome addition to the HALO® family of products, and an ideal tool for proteomic analysis.
REFERENCES:
-
Ghodsi R, Kobarfard F, Tabatabai SA. Application of narrow-bore HPLC columns in the rapid determination of sildenafil citrate in its pharmaceutical dosage forms. Iran J Pharm Res. 2012 Winter;11(1):123-7. PMID: 25317193; PMCID: PMC3876558.
-
R.P.W. Scott. An introduction to small-bore columns. J. Chromatogr. Sci. 23: 233–37 (1985).
-
5. K. Slais and D. Kourilova. Minimization of extra-column effects with microbore columns using
electrochemical detection. J. Chromatogr. 258: 57–63 (1983).
-
Cassidy L, Kaulich PT, Maaß S, Bartel J, Becher D, Tholey A. Bottom-up and top-down proteomic approaches for the identification, characterization, and quantification of the low molecular weight proteome with focus on short open reading frame-encoded peptides. Proteomics. 2021 Jun 19:e2100008. doi: 10.1002/pmic.202100008. Epub ahead of print. PMID: 34145981.



