AUTHOR: Stephanie Schuster, PhD, Senior Technical Support Scientist, Advanced Materials Technology 20 Years of High…

HALO® Fused-Core® Columns: Take Your UHPLC and HPLC Methods to the Next Level of Speed and Resolution
Introduction
A revolutionary innovation in liquid chromatography occurred in 2006 when Advanced Materials Technology introduced the first sub-3-µm superficially porous particles (SPP) and columns designed for HPLC and UHPLC of small molecules such as pharmaceuticals, industrial chemicals, and the like. These new SPP silica particles had 1.7 µm solid silica cores, which had a 0.5 µm layer of fully porous silica surrounding them. These new particles had an overall diameter of 2.7 µm and a porous layer that comprised 75% of the total particle volume. In addition, these novel particles had 90 Ångstrom pores ideal for small molecule analyses and produced comparable efficiencies and peak capacities to the recently-introduced 1.7 and 1.8 µm fully porous UHPLC columns, and showed very good loading capacity and backpressures that were 40–50% of the sub- 2-µm fully porous particle (FPP) columns under the same conditions.
Many of the reasons for the advantages of SPP columns were misunderstood and misconstrued early on before researchers were able to study them in more detail. Originally, some thought that the improved efficiency was due to shorter diffusion distances (0.5 µm vs. ~0.9 µm for FPP sub-2-µm columns) or due to the much narrower particle size distributions that resulted from the manufacturing process used to make SPPs. Others claimed that the very narrow superficially porous particle size distributions allowed SPPs to be packed more uniformly, leading to smaller van Deemter “A-term” values due to the more uniform analyte flow paths through the columns.
Subsequently, additional research into the performance of SPP columns produced the following commonly-accepted explanation for their higher efficiency: Much smaller reduced plate heights (h) and higher efficiencies for SPP columns due to a combination of smaller van Deemter A and B terms for SPP particles
- Reduction in eddy diffusion (40% smaller van Deemter “A term”) due to more uniform analyte flow paths through the column bed
- Much lower longitudinal broadening (25–30% smaller van Deemter “B term”), due to the presence of the particle’s solid core
- Flatter van Deemter plot and higher optimum linear velocity (μopt, ∝ flow rate) due to small van Deemter “C term” (resistance to mass transfer, μopt = √[B/C])
H=A+B/μ+Cμ
Equation 1. The van Deemter Equation Relating Theoretical Plate Height to Linear Velocity (μ), and Eddy Dispersion (A), Longitudinal Dispersion (B), and Resistance to Mass Transfer (C) Terms
For these reasons, HALO® Fused-Core® columns are able to perform comparably to sub-2-µm fully porous columns and substantially outperform 3 and 5 µm fully porous columns as seen in the van Deemter plots shown in Figure 1.
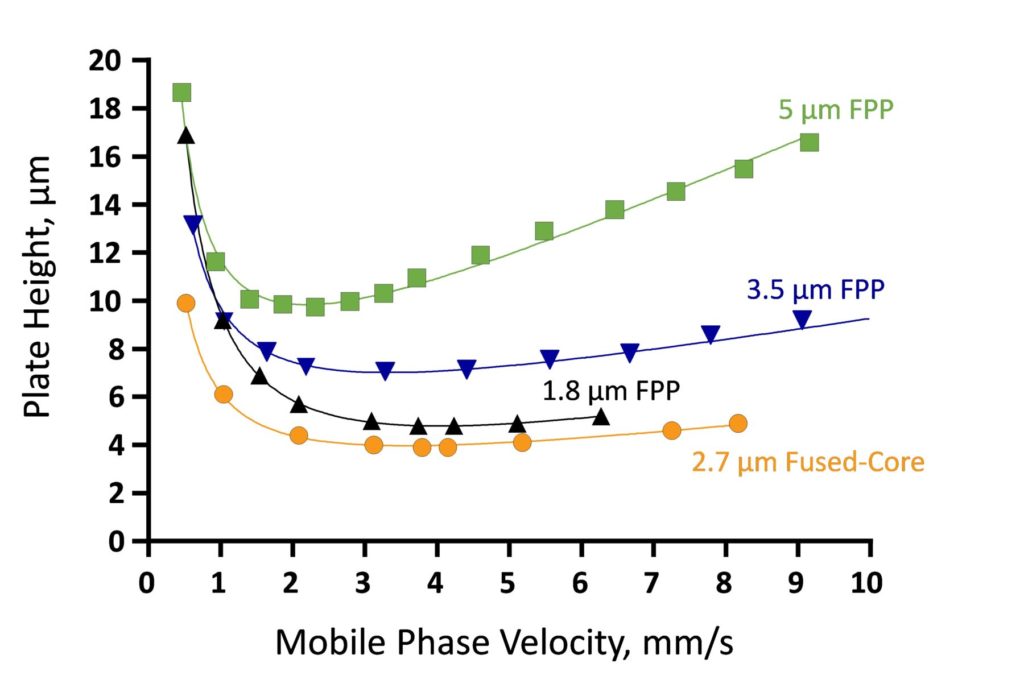
Figure 1. van Deemter Plots for 1.8, 3.5 and 5 µm Fully Porous Columns vs. 2.7 µm Fused-Core® Columns. All columns: 4.6 x 50 mm, 60/40 ACN/water, 24 °C.
Since the commercialization of the original 2.7 µm HALO® columns, Advanced Materials Technology has introduced additional products to the HALO® family for “small molecule” analyses: the 2 µm HALO® and 5 µm HALO® columns. The 2 µm HALO® columns were developed to provide additional resolving power for UHPLC systems, whereas the 5 µm HALO® product was designed to offer improved performance for pressure-limited HPLC systems and as a one-to-one replacement in older, fully porous particle (FPP) methods. 5 µm HALO® columns can deliver the efficiency of 3 µm fully porous columns at the pressures of 5 µm particles. The 2 µm and 2.7 µm HALO® columns are both suitable for use in UHPLC systems, with the 2.7 µm HALO® applicable with both UHPLC and HPLC instrumentation having 400 bar (5,802 psi) and 600 bar (8,702 psi) pressure limits.
The van Deemter plots for HALO® 5, 2.7, and 2 µm columns are presented in Figure 2, which shows that each of the three particle sizes has an optimum linear velocity range where maximum performance is obtained. As with fully porous particle columns, smaller SPP sizes tend to have flatter van Deemter plots (smaller C terms) and their respective plate height minima move to higher linear velocities (flow rates). These plots were obtained using 2.1 x 50 mm column geometries for the three HALO® particle sizes using naphthalene as a test probe under controlled, near-ambient temperatures.
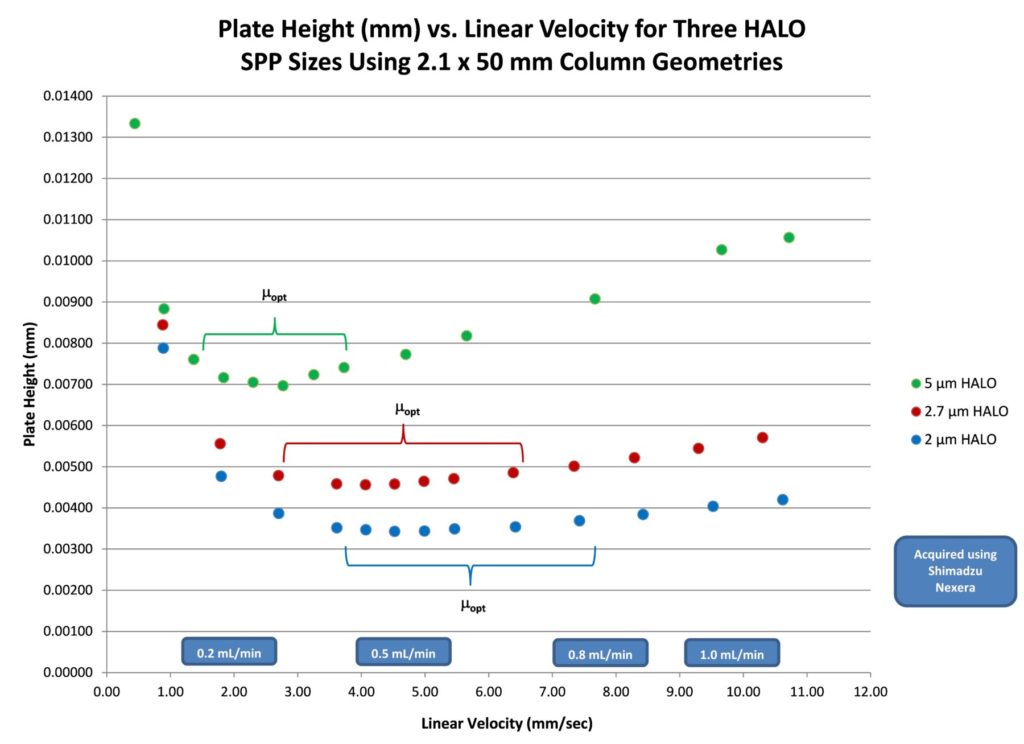
Figure 2. van Deemter Plots for 2 µm, 2.7 µm, and 5 µm HALO® Columns
HALO® Column Applications: Fast and High-Resolution Separations
The two main advantages of the innovative superficially porous HALO® columns are (1) increased analysis speed and throughput for 1-D separations and for the 2nd dimension of 2-D separations, and (2) improved resolving power for challenging samples. The availability of HALO® columns in particle sizes for both UHPLC and HPLC makes these advantages practical for those with instrumentation having 1000 bar (14,500 psi), 600 bar (8,702 psi), and 400 bar (5,802 psi) pressure limits.
Fast Separations HALO® columns can provide faster and higher resolution separations than larger particle size, fully porous particle (FPP) columns because Fused-Core® columns have lower total porosities (e.g., 0.5 vs. 0.65) and they have flatter van Deemter plots, so they can be used at higher flow rates with little or no loss in performance. Moreover, they can deliver comparable or higher efficiencies in shorter column lengths than in longer totally porous columns.
This first example (Figure 3) compares chromatograms for gradient separations obtained using a fully porous 4.6 x 250 mm column and a 2.1 x 100 mm, 2 µm HALO® RP-Amide column. The 100 mm 2 µm SPP column has a similar number of theoretical plates as the longer FPP column but can resolve this acid mixture in one-fourth the time with 12-fold less solvent consumption.
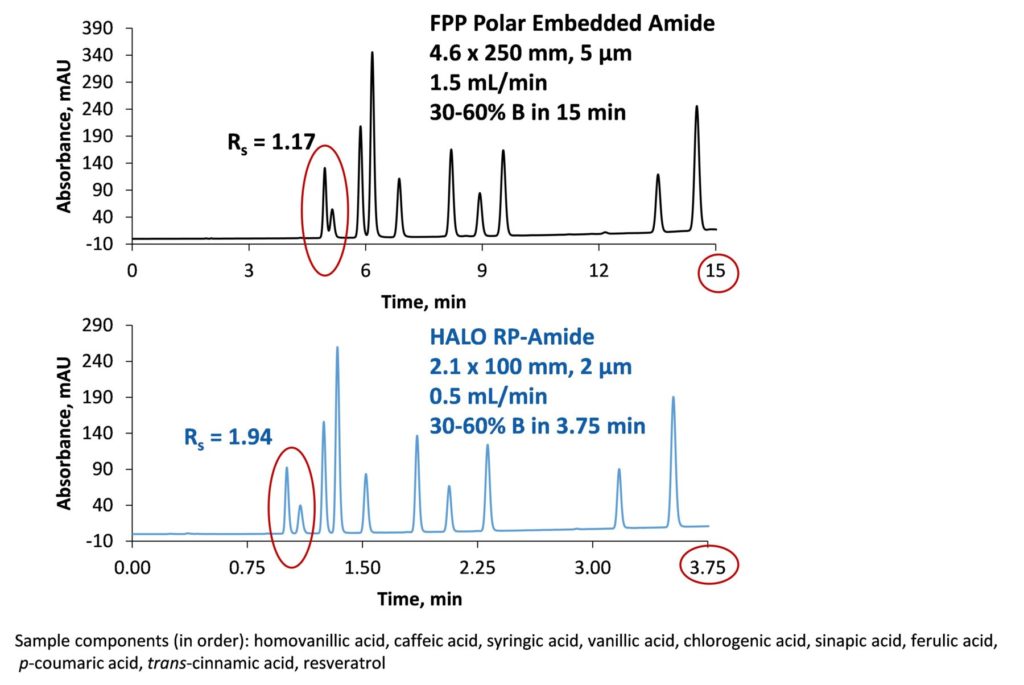
Figure 3. Faster Analysis Using a 100 mm, 2 µm HALO® Column vs. a 250 mm 5µm FPP Column
Ultrafast separations (Figure 4) are achievable using HALO® 2.7 and 2 µm columns, which can deliver comparable resolution and throughput compared to sub-2-µm columns at 50–80% of the pressure, respectively, or can be used at twice the flow rate (HALO® 2.7 µm) to provide 2-fold improvement in analysis time.
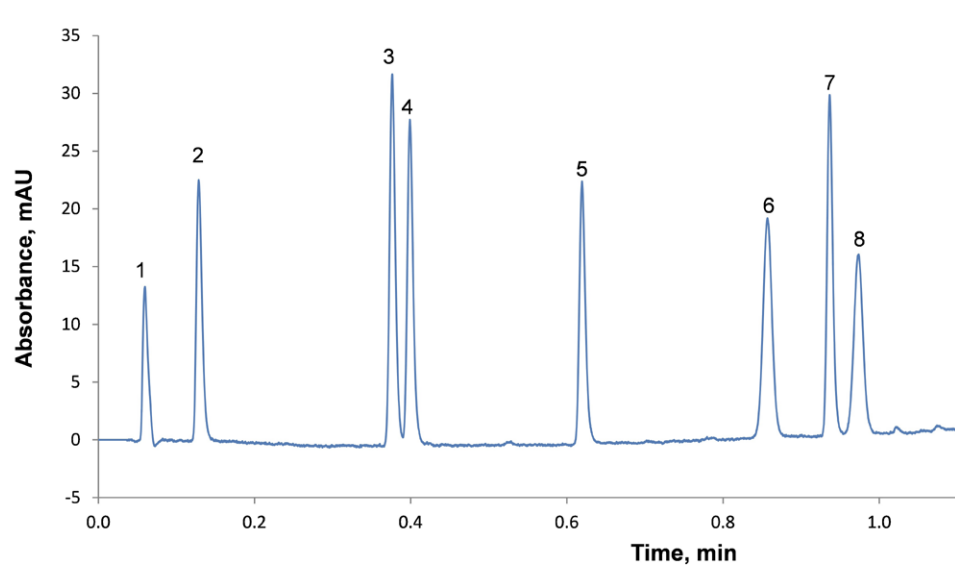
Figure 4. Ultrafast Separation of Anticoagulants Using a HALO® 2 µm, 2.1 x 30 mm Column
PEAK IDENTITIES
- Uracil (t0)
- 6,7-Dihydroxycoumarin
- 4-Hydroxycoumarin
- Coumarin
- 6-Chloro-4-hydroxycoumarin
- Warfarin
- Coumatetralyl
- Coumachlor
TEST CONDITIONS:
Column: HALO 90 Å C18, 2 µm, 2.1 x 30 mm
Mobile Phase A: 20 mM formic acid
Mobile Phase B: 50/50 ACN/methanol
Gradient: hold @ 20 %B until 0.06 min; 20-75 %B from 0.06-1.06 min
Flow Rate: 1.1 mL/min Temperature: 45 °C
Injection Volume: 0.2 µL
Detection: 254 nm, PDA
Performance versus Longer FPP 5 µm Columns
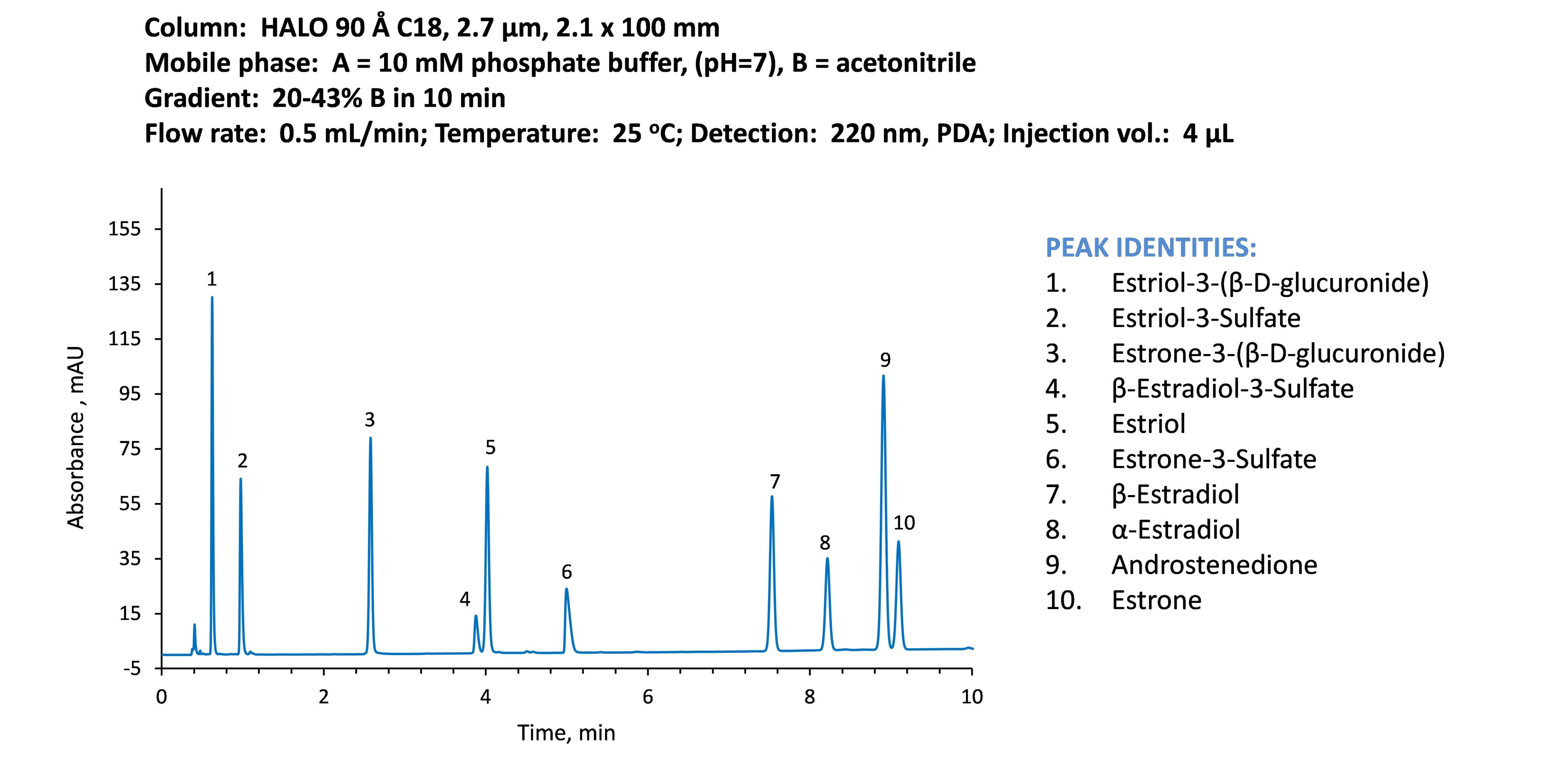
The next example (Figure 5) demonstrates the easy separation of ten steroids in less than 10 minutes using a 2.1 x 100 mm, 2.7 µm HALO® C18 column. This column length provides the efficiency and resolving power of a 250 mm fully porous 5 µm column but in a shorter time with much less solvent consumption and waste solvent generation. A comparable separation using a 5 µm, 250 mm column would take nearly 52 minutes at 1.25 mL/min.
Stationary Phase and Mobile Phase Screening for Systematic Method Development
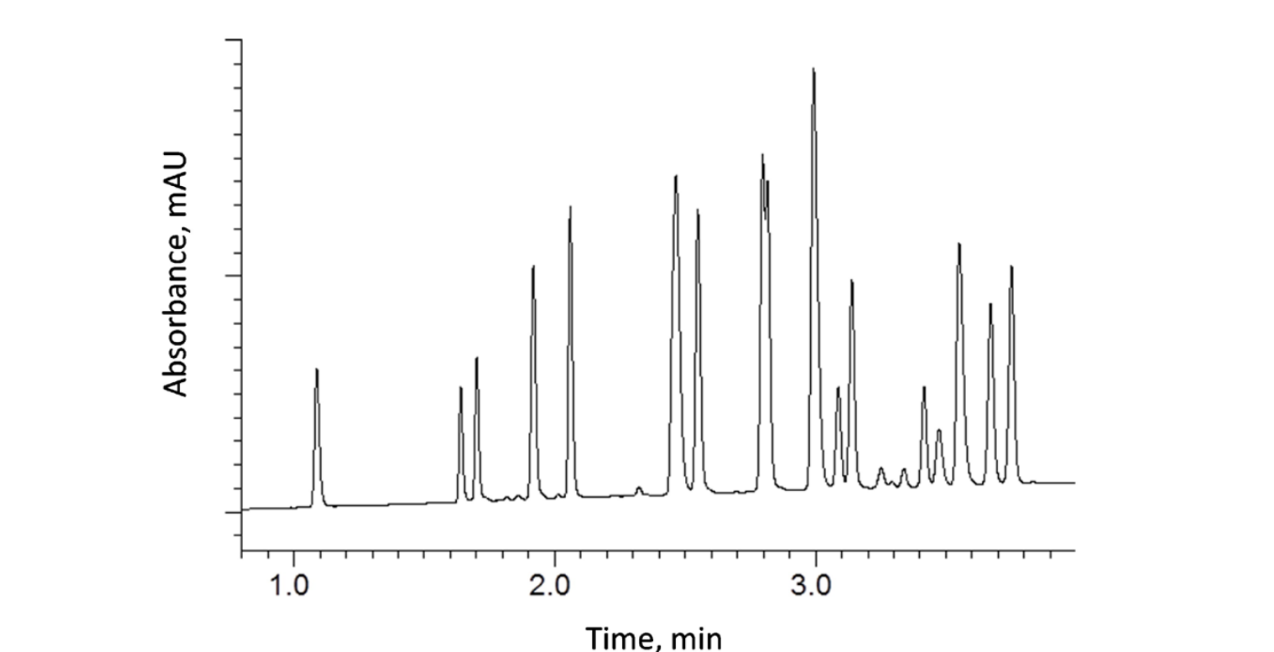
Another added benefit of using 2 µm HALO® and 2.7 µm HALO® columns in short 30 and 50-mm lengths is their applicability for column stationary phase and mobile phase screening during method development. In the following example, a 2.1 x 30 mm, HALO® 2 µm RP-Amide column was used to separate 19 out of 20 flavonoids in under 4 minutes. These small, very efficient column geometries allow the comparison of various combinations of stationary phase, organic modifier, and mobile phase pH in a much shorter amount of time than using conventional fully porous columns.
TEST CONDITIONS:
Gradient: 5-90% CH3CN/water with 0.1% HCOOH, in 6.1 min
Flow Rate: 0.6 mL/min
Temperature: 25 °C
Instrument: Agilent 1200 SL
5 µm HALO®: Improved Throughput and Better Efficiency for HPLC Separations
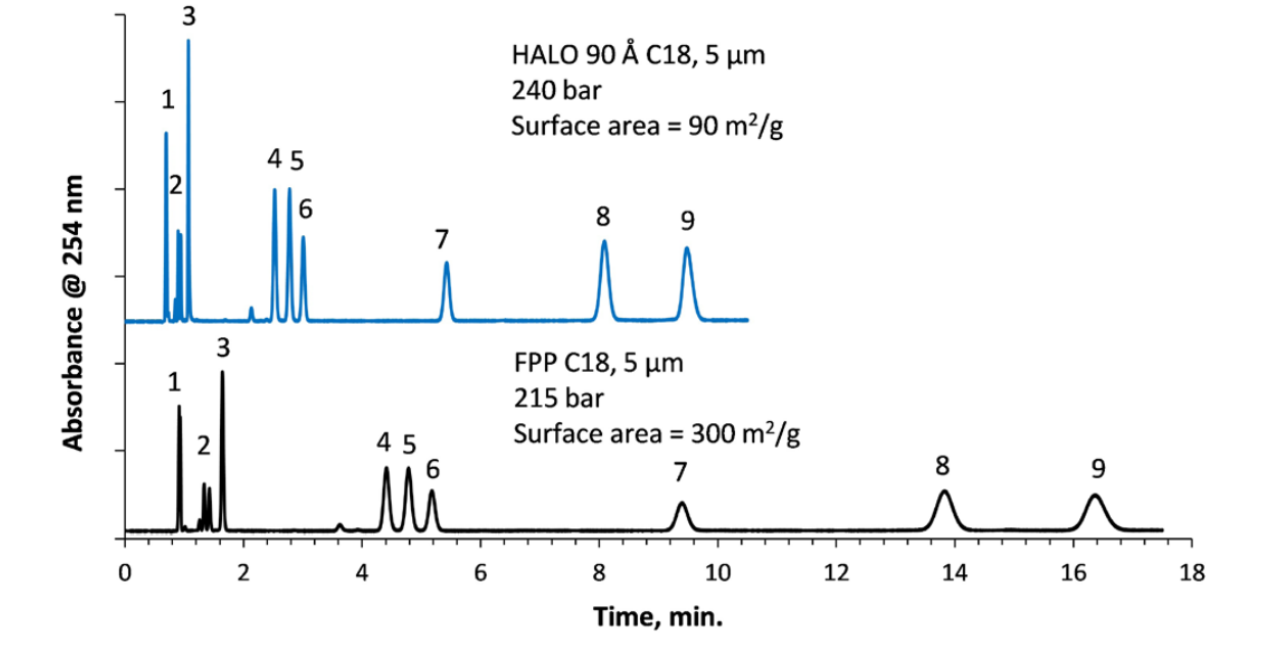
For HPLC separations, significant improvements in analysis time and resolution can be achieved using HALO® 5 µm SPP columns as shown in Figure 7.
In this example, efficiencies in this isocratic separation using a 4.6 x 150 mm, HALO® 5 µm column are higher by 60–85%, and run time is shorter by ~40% compared to the 5 µm FPP column.
PEAK IDENTITIES
- Acetaminophen
- Aspirin
- Salicylic acid
- Tolmetin
- Ketoprofen
- Naproxen
- Fenoprofen
- Diclofenac
- Ibuprofen
TEST CONDITIONS:
Column: 4.6 x 150 mm
Mobile Phase A: 20 mM potassium phosphate (pH = 2.5)
Mobile Phase B: 50/50 ACN/methanol
Isocratic: 48/52 A/B
Flow Rate: 2.0 mL/min
Temperature: 35°C
Injection Volume: 2 µL
Detection: 254 nm, UV
Compound |
HALO® Plates |
FPP Plates |
|
Naproxen |
16,400 |
10,000 |
|
Ibuprofen |
20,500 |
11,000 |
High-Throughput LC-MS Analyses
Those conducting high-throughput LC-MS analyses have found another benefit for HALO® columns. 2 µm particle columns provide increased resolution and speed to optimized systems while the 2.7 µm particle columns offer a forgiving compromise, and 5 µm particle columns offer slightly broader peaks which are ideal for instrumentation with slower LC-MS scan speeds that cannot handle the much higher backpressures and very small peak widths and volumes produced using sub-2-µm fully porous columns.
High-Resolution Separations for Complex Samples
In addition to their capability to provide very fast separations, another significant advantage of HALO® Fused-Core® columns is that they can produce high-resolution separations in both isocratic and gradient modes. This sample of dinitrophenylhydrazine-derivatized carbonyl compounds (Figure 8) shows excellent resolution of the individual derivatives and the corresponding isomers for the asymmetric aldehydes in the mixture in approximately 25 minutes at ~820 bar (11,890 psi) using a 150 mm HALO® 2 µm C18 column. Complex samples can also be readily separated at pressures compatible with HPLC instrumentation using HALO® 5 µm columns. In Figure 9, a separation of carbonyl compound DNPH derivatives, which required a pressure of 856 bar (12,412 psi) and a UHPLC
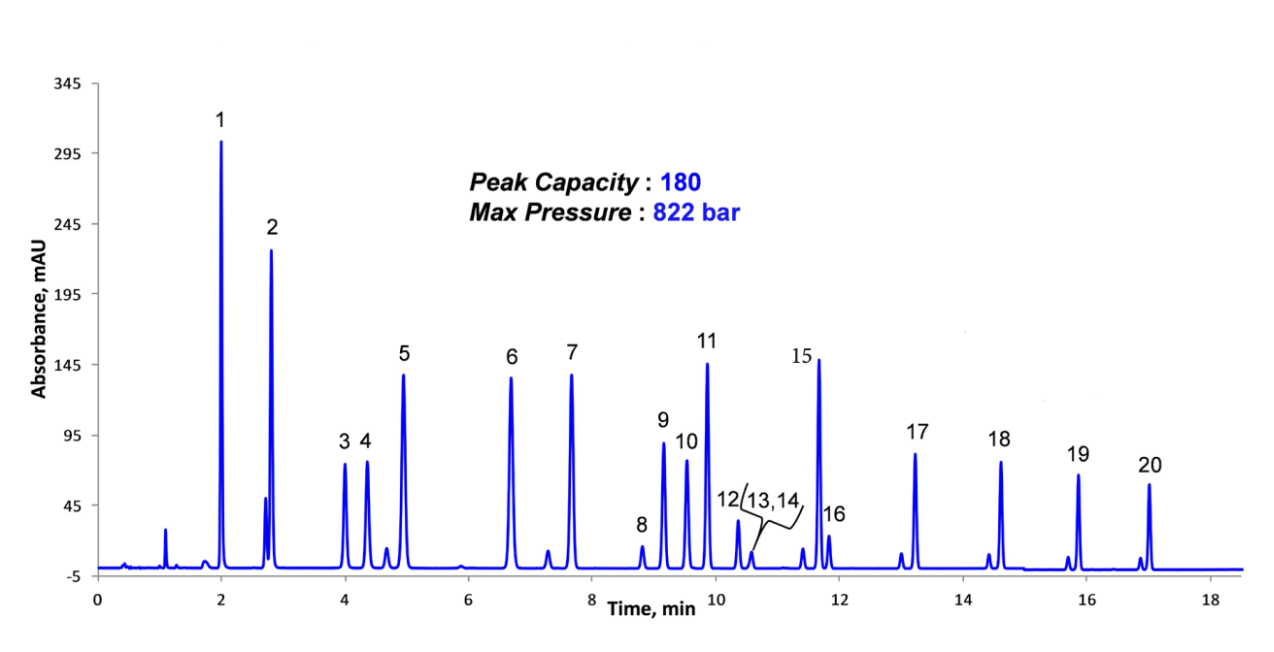
Figure 8. Gradient Separation of the DNPH Derivatives of 20 Carbonyl Compounds using a 150 mm HALO® 2 µm Column
PEAK IDENTITIES*
- Formaldehyde
- Acetaldehyde
- Acetone
- Acrolein
- Propionaldehyde
- Crotonaldehyde
- Butyraldehyde
- Benzaldehyde
- Cyclohexanone
- Isovaleraldehyde
- Valeraldehyde
- o-Tolualdehyde
- m-Tolualdehyde
- p-Tolualdehyde
- Hexaldehyde
- 2,5-Dimethylbenzaldehyde
- Heptaldehyde
- Octyl aldehyde
- Nonanal
- Decyl aldehyde
* All compounds are 2,4-DNPH
TEST CONDITIONS:
Column: HALO 90 Å C18, 2 µm, 2.1 x 150 mm
Mobile Phase A: water
Mobile Phase B: 80/20 ACN/tetrahydrofuran
Gradient: hold @ 45 %B 0-5 min, 45-90 %B 5-20 min
Flow Rate: 0.5 mL/min
Temperature: 30 °C
Injection Volume: 1 µL
Detection: 360 nm, PDA
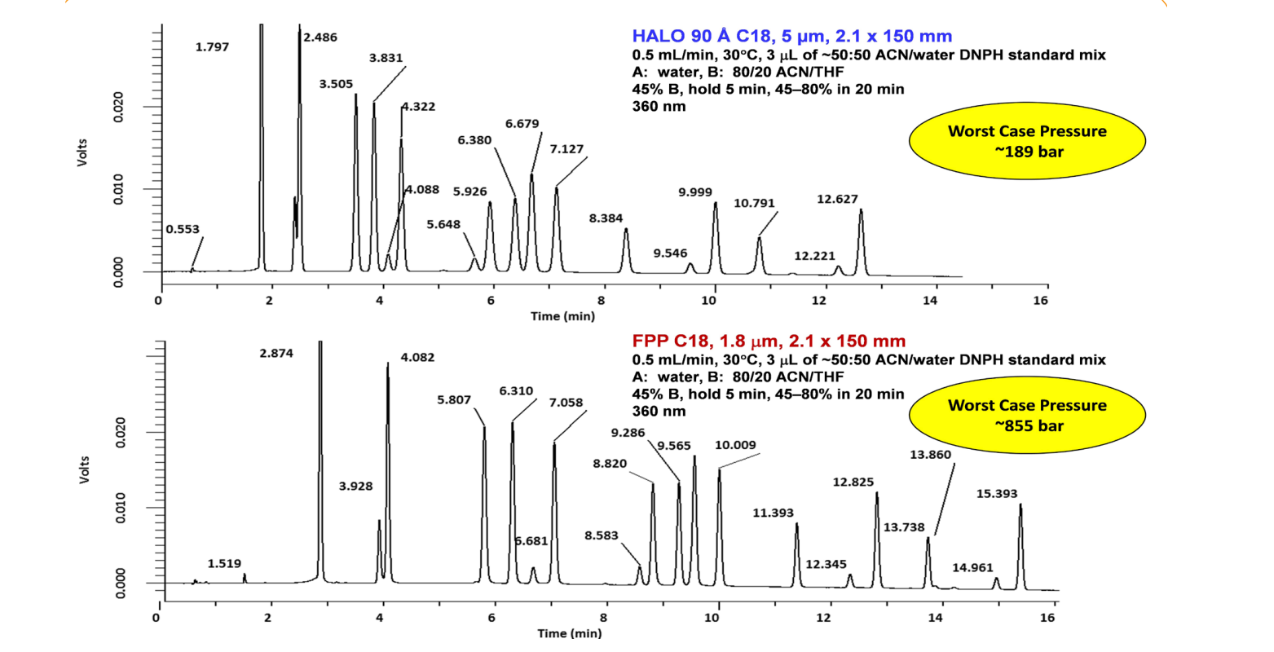
Figure 9. Comparison of Separations of Carbonyl DNPH Derivatives Using 5 µm HALO® and 1.8 µm FPP Columns Under the Same Conditions
PEAK IDENTITIES*
- Formaldehyde
- Acetaldehyde
- Acetone
- Acrolein
- Propionaldehyde
- Crotonaldehyde
- 2-Butanone
- Methacrolein
- Butyraldehyde
- Benzaldehyde
- Valeraldehyde
- m-Tolualdehyde
- Hexaldehyde
* All compounds are 2,4-DNPH
Ultrahigh Resolution Using Columns in Series
Another significant advantage that the HALO® 2.7 µm and 5 µm columns afford, due to their lower backpressure and high efficiency, is that long single columns or multiple long columns in series can be used to produce ultrahigh-resolution for complex samples. In fact, it has been demonstrated that perdeuterated or partially deuterated analogs can be separated to baseline in isocratic mode when multiple columns are used in series.
In Table 1, the theoretical plate counts, expected resolution, and back pressures are estimated for single columns, and for two or three columns in series, for both HALO® 2.7 µm and HALO® 5 µm columns in 150 and 250 mm lengths. These estimated pressures were calculated for a worst-case viscosity of an acetonitrile/water mixture with a flow rate of 1.0 mL/ min at 40 °C for 4.6 mm ID columns. Although efficiency and pressure double with each additional column, resolution increases only by √N. Nevertheless, for challenging separations, the ability to generate 50,000 to 100,000 plates is a significant advantage that HALO® Fused-Core® columns can provide for both difficult UHPLC and HPLC separations.
Table 1. Comparison of Theoretical Plate Counts and Estimated Backpressures for Various HALO® 2.7 µm and HALO® 5 µm Columns (Alone or in Series)
|
# Columns in Series |
Total Length (mm) |
ID (mm) |
Particle Size (µm) |
Reduced Plate Height (h) |
Predicted Ncol |
Resolution (Rs) |
Estimated Pressure (bar) |
|
1 |
250 |
4.6 |
2.7 |
1.4 |
66,100 |
1.9 |
334 |
|
2 |
500 |
4.6 |
2.7 |
1.4 |
132,000 |
3.9 |
668 |
|
3 |
750 |
4.6 |
2.7 |
1.4 |
198,000 |
5.8 |
1002 |
|
1 |
150 |
4.6 |
2.7 |
1.4 |
39,700 |
1.5 |
200 |
|
2 |
300 |
4.6 |
2.7 |
1.4 |
79,400 |
2.1 |
400 |
|
3 |
450 |
4.6 |
2.7 |
1.4 |
119,000 |
2.6 |
600 |
|
1 |
250 |
4.6 |
5 |
1.3 |
41,800 |
1.9 |
115 |
|
2 |
500 |
4.6 |
5 |
1.3 |
83,600 |
3.9 |
230 |
|
3 |
750 |
4.6 |
5 |
1.3 |
125,400 |
5.8 |
345 |
|
1 |
150 |
4.6 |
5 |
1.3 |
25,100 |
1.5 |
69 |
|
2 |
300 |
4.6 |
5 |
1.3 |
50,200 |
2.1 |
138 |
|
3 |
450 |
4.6 |
5 |
1.3 |
75,300 |
2.6 |
207 |
TEST CONDITIONS:
1 mL/min, 40 °C, worst case viscosity (10-20% v/v) for CH3CN/water (pressure drop column only)
Resolution normalized relative to the resolution of 1.5 for a 150 mm length and plate counts for both HALO® 2.7 µm and HALO® 5 µm
Importance of Extra-column Dispersion
When HALO® 2.7 µm Fused-Core® columns and sub-2 µm totally porous columns were introduced in the mid-2000s, it was clearly demonstrated that these highly efficient columns needed instrumentation that had low extra-column dispersion so that maximum performance could be obtained. Considerable efforts were made to understand and describe how various instrument configurations and method parameters affected column efficiency.
Unfortunately, the importance of extra-column volume and extra-column dispersion was not well understood by most HPLC users at that time. Most chromatographers had only used 4.6 mm ID columns, and few had experience with narrower ID columns (2.1, 3.0 mm) in short, 50–150 mm lengths. Instrumentation that worked well with longer (250 and 150 mm) or wider (4.6 mm ID) 3 and 5 µm fully porous columns did not have sufficiently low extra-column volume and dispersion for the optimal use of 2.7 µm SPP columns—or for the sub-2-µm fully porous columns commercialized in 2003 and 2004. Among the various instrument and method options and settings, the following parameters (Table 2) have been shown to be most important for minimizing extra-column dispersion for conventional instrumentation. Recommendations are also given for the selection of appropriate choices for each of the parameters.
Table 2. Recommendations for Instrument and Method Parameters That Affect Extra-Column Dispersion and Maximize Column Performance
|
Instrument or Method Parameter |
Recommendations |
|
Injection Volume |
|
|
Sample Solvent Composition |
|
|
Pre-column Tubing Volume |
|
|
Heat Exchanger Volume |
|
|
Post-column Tubing Volume |
|
|
Detector Flow Cell Volume |
|
|
Detector Data Rate |
|
|
Detector Response Time |
|
Most new UHPLC instrumentation (600–1300 bar (8,702-18,855 psi) maximum pressure) is configured for low extra-column volume and dispersion, or there are available add-on kits that allow you to modify your instruments for low dispersion. An updated technical note will be available in the near future, which will describe in detail how to measure extra-column dispersion for your instrument.
The following table provides general guidance regarding which instruments and corresponding pressure limits are most applicable for the use of HALO® 5, 2.7, and 2 µm columns.
Table 3. Instrument Pressure Limits For 5, 2.7, and 2 µm HALO® Columns
|
INSTRUMENT PRESSURE LIMIT |
|||
|
HALO® Column |
HPLC 400 bar1 |
HPLC/UHPLC 600 bar2 |
UHPLC 1000–1300 bar3 |
|
5 µm |
• |
• |
• |
|
2.7 µm |
• |
• |
• |
|
2 µm |
X |
• |
• |
1HPLC instruments typically need to be reconfigured for lower extra-column volume and dispersion.
2Some instruments need to be reconfigured for lower dispersion and only shorter 2 µm HALO® columns are applicable.
3Most or all UHPLC instruments are configured for low dispersion or can be modified for even lower dispersion.
Summary
HALO® Fused-Core® columns were a major innovation when they were first commercialized by Advanced Materials Technology (AMT) in 2006, and brought a revolutionary change to the HPLC and UHPLC column markets. HALO® particle technology has evolved into three particle sizes for UHPLC and HPLC small molecule analyses: 2 µm, 2.7 µm, and 5 µm. They can deliver both fast and high-resolution separations that allow chromatographers to choose the best combination of particle size and column geometry for their methods and their instrumentation. The adoption of 2 µm HALO® particles using appropriate instrumentation provides more separation power than previously available. HALO® Fused-Core® columns deliver the desired performance for a variety of applications from ultrafast high-throughput separations of 96–384 well plates to ultrahigh resolution separations of impurity profiles for new and generic pharmaceuticals, complex environmental sample separations, and challenging LC-MS separations.
HALO® and Fused-Core® are registered trademarks of Advanced Materials Technology, Inc.



