The goal for chromatographic separations is to achieve well resolved peaks with adequate retention and…

LC Chromatography Troubleshooting
Introduction
High-pressure liquid chromatography (HPLC) is a benchmark technique used in laboratories across the world. However, even experienced analysts that are familiar with HPLC concepts and principles can fall victim to chromatographic pitfalls that arise. Some common issues include misshapen peaks, unstable baselines, problems with reproducibility, and the observance of irregular back pressure. Identifying and correcting these problems quickly can not only save time but increase productivity in the lab. Chromatographic issues can arise from any part of the system, including the pump, autosampler, column oven, or detector, and each component of the system must be considered individually.
The Pump
It is important to use high-grade solvents, especially when using a mass spectrometer to reduce contamination. Buffers should be changed out on a regular basis (typically 1-3 days) and HPLC grade water should be used. There are several components of the pump that can cause chromatographic issues including a worn-down pump seal, or even a plugged check valve. These issues can lead to inaccurate or nonrepeatable retention times, unstable back pressure, or the inability to operate.
The Injector/ Auto Sampler
The injector/ autosampler is used to introduce the sample into the LC. A scratched valve rotor or even a plugged needle could cause issues with peak shape and area, as well as double peaks for all analytes. It is important to filter sample solutions when necessary to prevent issues within the autosampler and to protect the analytical column from plugging.
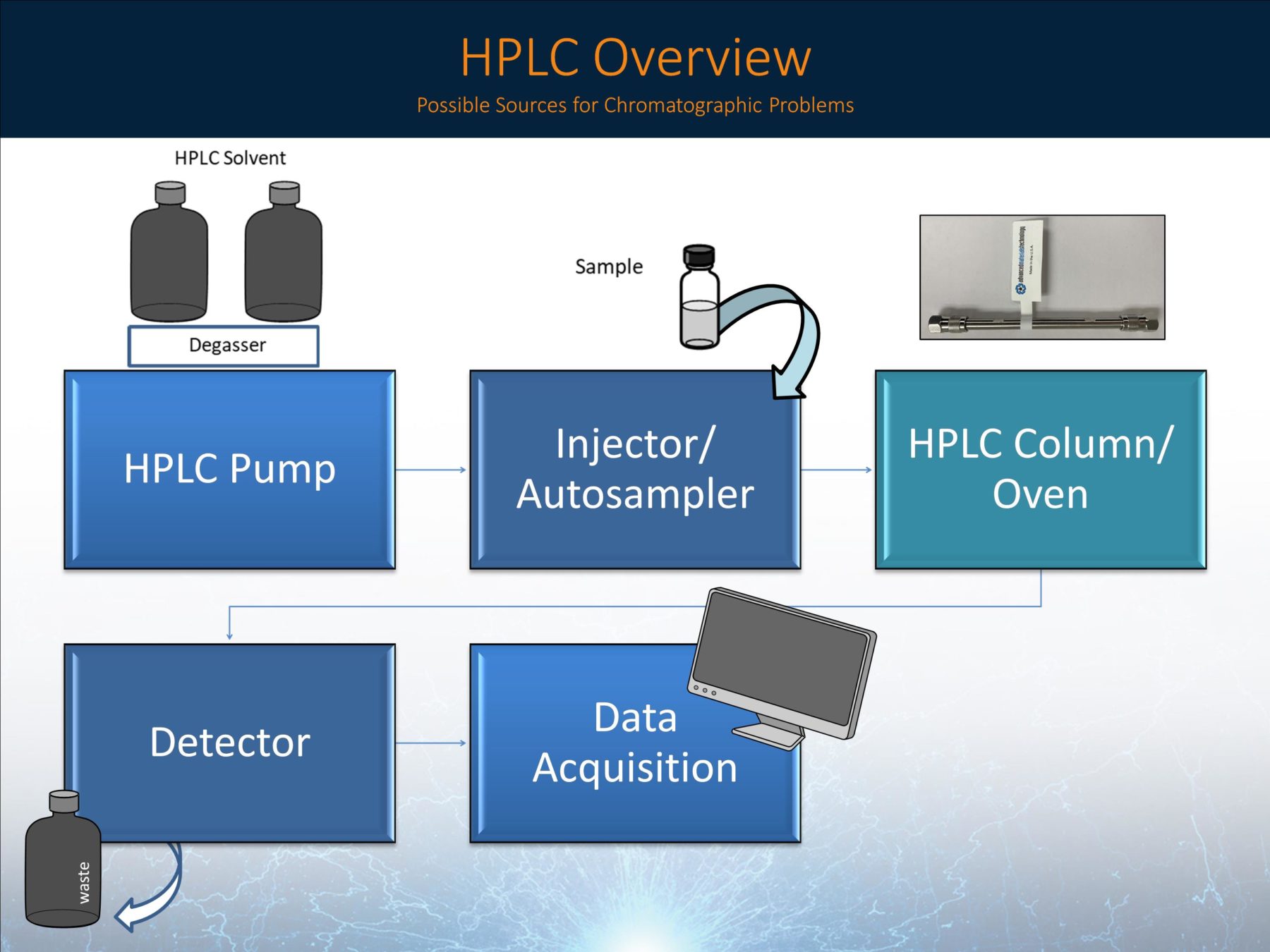
Figure 1: HPLC Overview (and possible sources for chromatographic issues)
The Column Oven and Column
Some LC systems include a column oven compartment, which maintains a constant column temperature. Having consistent column temperature ensures retention time repeatability (within a day) and reproducibility (day-to-day) and ensures that selectivity (relative peak retention) will not be affected by inaccurate or inconsistent column temperatures. Major temperature differences between the mobile phases and column oven can also lead to irregular and broadened peaks. Heat exchangers are very often used within the column oven so that mobile phases are close to the column temperature, which helps to reduce these effects.
The column, which performs the separation, can also be the cause of HPLC issues. However, columns are consumables, and they may eventually become plugged or contaminated and will need to be replaced. Guard columns and in-line filters can also be used to help increase the column lifetime.
UV-Visible or Refractive Index Detector
A bad lamp or contaminated flow cell causes issues such as poor signal, or irregular baseline. It is also important to check your detector settings as they can significantly change your column efficiency if the data acquisition rate is too slow. If a concentrated sample is introduced to the system, the detector signal can also become saturated leading to flat top and broad peaks.
Human Error
Human error is one of the biggest sources of chromatographic issues. Poor sample preparation, inadvertent contamination of mobile phases, or even a poor fitting connection can all lead to chromatographic issues. It is important to keep all these items in mind when troubleshooting HPLC problems. Knowing where to start and what to do will be discussed in the next several topics.
Peak Broadening
As chromatographers, we are constantly trying to get sharp, efficient peaks and baseline resolution for our separations. Peak broadening can prevent this and can be caused by a variety of reasons. Several possible causes for peak broadening are shown in Figure 2. Mobile phase temperature and sample solvent strength mismatch are also possible causes that may need to be investigated.
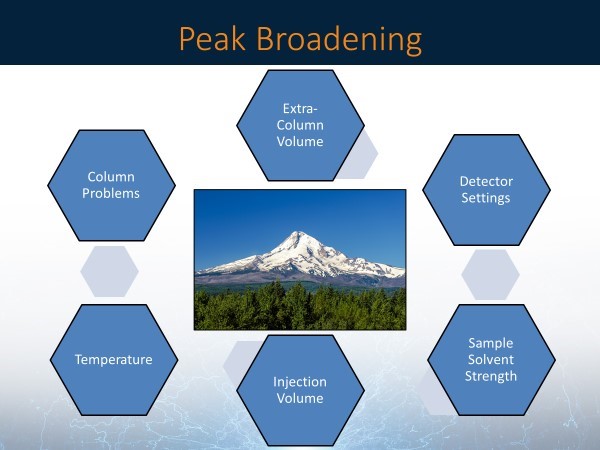
Figure 2: Some possible causes of peak broadening
Extra Column Volume
Extra column volume, the volume in an HPLC system external to the column that contributes to the total peak volume can impact peak width, efficiency, and overall resolution. Most new UHPLC chromatographic systems have been optimized for low extra column volume and dispersion, and are able to use a high efficiency, superficially-porous-particle (SPP) and core-shell columns, but many older liquid chromatographs are still in active use and may have to be retrofitted or it may be necessary to use larger volume SPP columns with larger particles (e.g., 3 and 4.6 mm ID columns in 75-250 mm lengths with 5 µm particles (HALO 5).
Loss in efficiency and peak broadening can be problematic for many reasons. This efficiency loss is a function of both external and internal column effects. There are several quick and simple external column changes that could significantly improve your chromatographic separations and should be considered by every chromatographer.
The following should be considered to reduce extra column volume:
- Keep injection volume to a minimum
- Keep sample solvent strength equal to or less than the initial mobile phase
- Heat exchanger volume (if used) should use the smallest volume consistent with the flow rates
- Use minimum lengths of smaller ID tubing for pre and post-column connections
- Reduce the detector flow cell volume
Detector Settings
Detector settings should also be considered when noticing broad peaks and low efficiency. This could be a quick and easy change that could make a significant difference. For example, figure 3 shows a separation of neutral compounds at different data acquisition rates. Peak efficiency improves significantly as the data rate setting is increased. It is recommended to have at least a 20 Hz acquisition rate in your detector settings.
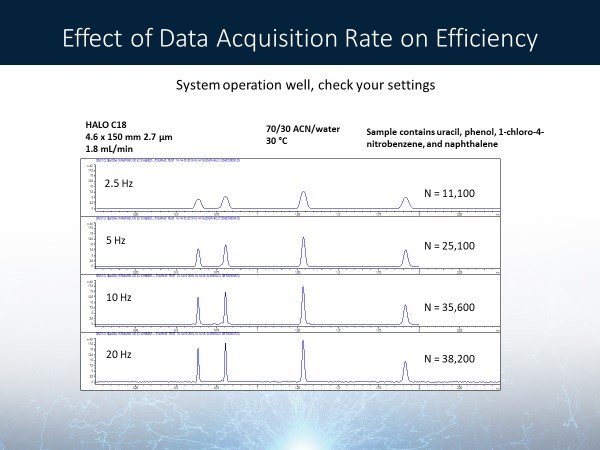
Figure 3: Effect of Data Acquisition Rate on Efficiency
Sample Solvent Strength
Try to ensure that your sample solvent is as weak or weaker than the initial mobile phase being used for your analysis. Using too strong a solvent or too large a volume of too strong a sample solvent can cause peak shape and efficiency problems. Sample solvent mixtures that have different viscosities than the initial mobile phase can cause peak shape issues as well.
Sample Overloading
It is important to know how much sample can be introduced onto the column before the loss in performance is observed. When scaling methods to a smaller diameter column, sample injection should also be reduced proportionally to the ratio of the column dead volumes. This calculation should use the predicted column dead volumes incorporating the porosity of the packing materials (HALO, ~0.5 for 90 Ångstrom and 0.54 for 160 Ångstrom pore sizes).
Column Issues
Poor efficiency and poor peak shape can also be caused by the column if it becomes fouled with particulates or strongly retained materials. Proper filtering of sample solutions and the use of a guard column and/or a pre-column filter is recommended to try to avoid problems with the column frit and column bed. It is important to read the care and use sheets for each column to be aware of pH, temperature, and back pressure limitations. Cleaning and storing the column properly can also increase column lifetimes. Column voids can also occur from significant pressure shocks from injections or from column mishandling and can lead to split peaks.
Baseline Issues
Problems with the UV baseline can be from several things, but usually problems with the flow cell or lamp. There are many different baseline trends that can be observed and the most common three will be covered.
- Baseline Spikes: If baseline spiking is observed, it is recommended to purge the system. If the problem continues clean the system with water, isopropanol (2-propanol), followed by water again. The goal is to get any air out of the system, as air bubbles can cause baseline spiking. A good clue that the problem is from the air is if the problem only exists while the mobile phase is flowing through the system. If the problem occurs with no flow, replacement of the lamp may be necessary.
- Baseline Drift: Negative or positive drift in the baseline is usually caused by absorbance differences caused by concentration changes in one or both components of the mobile phase in a gradient run. If drift occurs, an additive may need to be added to the mobile phase modifier or a different buffer or additive may need to be used. If that does not solve the problem, the detection wavelength may need to be changed. For example, a gradient separation with trifluoroacetic acid (TFA) only in mobile phase B (ACN) is compared to a separation in which no TFA is used in mobile phase A or B (water and ACN, respectively) is shown in Figure 4.
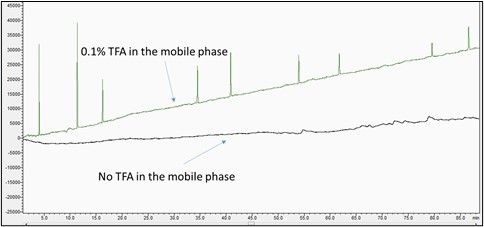
Figure 4: Baseline drift from acidic modifiers in the mobile phase
- Noisy Baseline: A noisy baseline could be an indication of a bad lamp. It is important to keep track of your lamp hours to have a general idea of when replacement is necessary. Lamps have a shelf life as well, so it is important to purchase one when necessary. A dirty flow cell could also cause a noisy baseline. Flushing the HPLC system with isopropanol (entire flow path including flow cell) or even passivation of the system may be required. If the problem still exists, a new flow cell may be needed.
Artifact Peaks
Unknown peaks in the separation could be due to artifacts from the system. There are several reasons why unknown peaks can occur, and there are some troubleshooting tips to try when an artifact peak is observed. There are four main types of artifact peaks: late elution peaks, carry-over peaks, contamination, and ghost peaks (Figure 5).
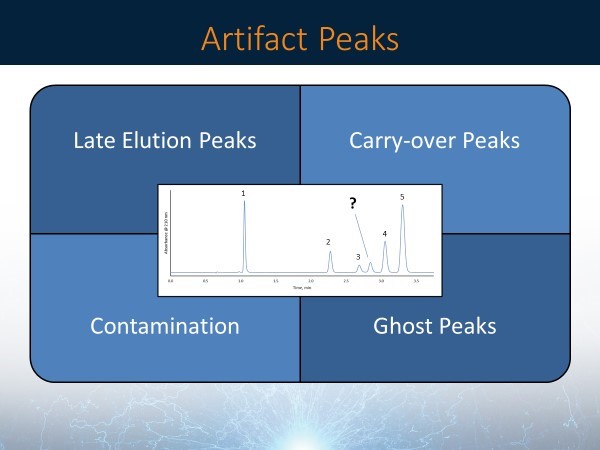
Figure 5: Common causes for artifact peaks
- Late Elution Peaks: Make sure that the run time is long enough, especially when new separations are being developed or a new sample type with unknown or unexpected components is being analyzed for the first time. If there is a broad peak in the chromatogram that is not consistent with the width of nearby peaks, it could be a late eluting peak from a previous injection. A late-eluting peak can often occur for isocratic separations. Simply extend your run time to see if this is the case. For new samples, having a long run time is better than having too short. For unknown samples, it’s wise to carry out a broad range gradient (e.g., from 5-95% organic modifier, buffer solubility permitting) to ensure that all sample components elute from the column.
- Carry-over Peaks: If the retention time of the artifact peak is the same as the retention time of the main peak, sample carry-over could be the problem. Some samples will remain in the system longer than most and could get stuck on the needle or elsewhere in the system. To avoid carry-over peaks, rinsing the internal/ external part of the needle before and after injections may need to be performed. A series of blank injections may also need to be done using a 100% organic modifier as the sample.
- Contamination (sample): If extending the run time and running a series of blank injections does not remove the artifact peak, a new sample may need to be made. Many samples degrade over time and can decompose or react to yield other components. For example, Figure 6 shows the comparison of chromatograms from the analyses of a freshly prepared sample and an older sample. The old sample shows a decrease in the area of the main peak and artifact peaks come up at a different retention time compared to the main peak.
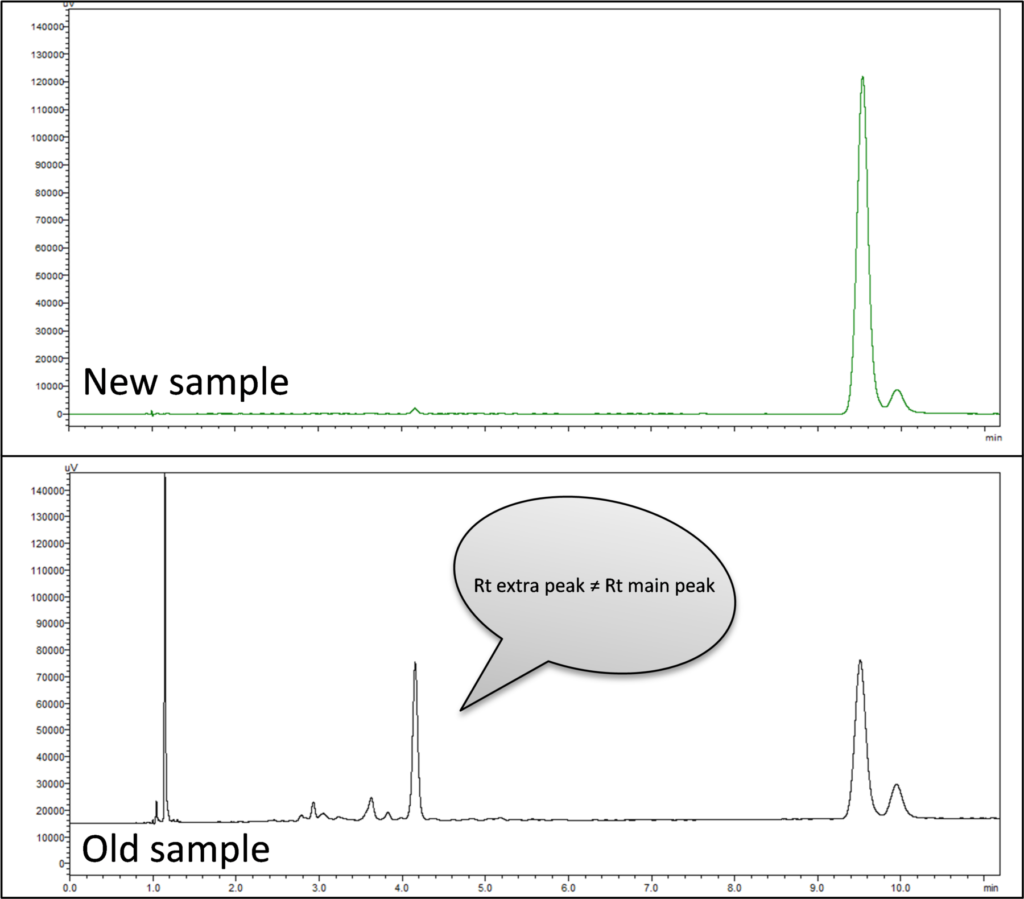
Figure 6: Artifact Peak: Difference between a freshly prepared sample and an older sample
- Ghost Peaks (Mobile Phase Contamination): If there is still an artifact peak after extending the run time, running a series of blank injections, and using a fresh sample, the mobile phases may be compromised. It is important to use HPLC/ MS grade solvents along with HPLC-grade water. If your solvents are running low in your reservoirs, avoid topping them off, and instead, empty them completely followed by cleaning the bottles. Keep in mind that mobile phase modifiers can also become contaminated. For example, Figure 7 shows a comparison of chromatograms using a mobile phase with and without contaminated formic acid, which produced a ghost peak in the middle of the chromatogram from the contaminated additive.
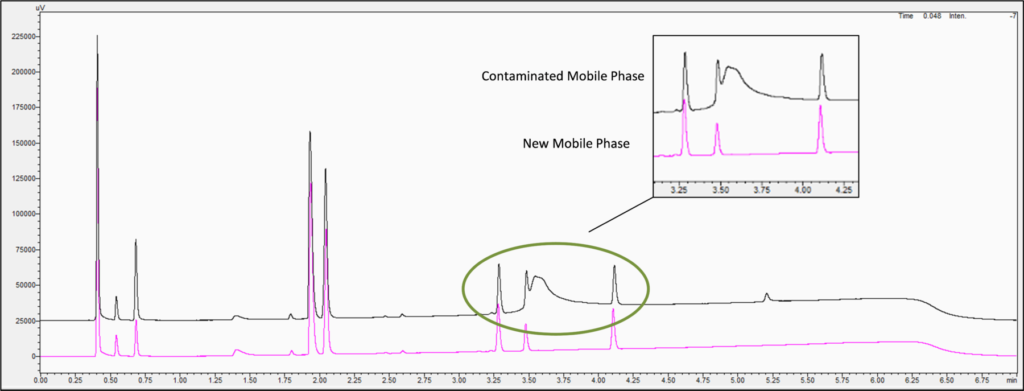
Figure 7: Ghost peak caused by formic acid contamination in the mobile phases
Peak Area Variability
Peak area variability can be very problematic especially when quantification is required. There are several trends observed with inconsistent area counts such as peak areas decreasing over time, random peak areas, or peak areas increasing over time.
- Peak Area Decreasing: A gradual decrease in area count could be caused by a sample degrading over time. If necessary, a temperature-controlled autosampler can be used to minimize or prevent degradation. A sudden decrease in the area for a given run or run could mean that there is a leak in the system, usually in the autosampler. Check fittings for micro leaks and tighten down fittings with a wrench. A complete loss in the area usually is caused by an air bubble in the sample vial or no sample present in the vial.
- Peak Area Random: Random area counts could mean that the rotor/ stator in the autosampler is scratched. Inspection of these items should be done and replaced if necessary.
- Peak Area Increasing: A gradual increase in area count could be from column-sample interaction, especially for biomolecules greater than 10,000 Daltons. Column conditioning is recommended, which will often improve column performance by binding active adsorption sites. Multiple injections of a higher concentration than normal of a large protein such as BSA (bovine serum albumin) onto the column can help saturate these active sites.
Retention Time Variability
Retention time variability on a specific instrument can happen for several reasons. One test to try to make sure that the pump is working properly is a flow rate test. If the flow is not accurate or consistent, checking for leaks is recommended as a pump seal may have gone bad. A standard column (one dedicated for your system test or for a given method) is highly recommended to test on a regular basis to track sudden pressure and retention changes on your system. Monitoring things such as retention time and back pressure can help troubleshoot issues much more quickly
Instrument Changes
If analyte retention times differ between one instrument and another, make sure that the dwell volume (gradient delay volume) on both instruments is the same. The dwell volume is the volume of the gradient mixer plus that of the mobile phase flow path between the mixer and the column inlet. Differences in the dwell volume between instruments will lead to retention time differences and may cause selectivity differences for gradient separations. Differences in extra column volume (different tubing lengths, different tubing IDs, different volume heat exchangers, etc. in the sample flow path) will also cause retention time differences.
Same Instrument
Retention time variability between the same column and the same instrument can be caused by several different factors. First, make sure your mobile phases are fresh and prepared the same each time on the same day or on different days. If a specific pH is required for the mobile phase, ensure that your pH meter has been calibrated recently. If a temperature-controlled oven is not in use, try to ensure that the laboratory temperatures are well-controlled. The mobile phase, pH, buffer, and temperature changes all can affect the repeatability and reproducibility of retention time and selectivity (relative retention).
Column Changes
Differences in retention time between different columns can also occur. Keep in mind that columns are consumables, and in general, over time they will tend to lose retention. Follow good practices that will maintain and extend column performance and lifetime. When validating a chromatographic method, lot-to-lot reproducibility experiments (ideally, 3 or more lots) should be performed to make sure that retention times and selectivity are not affected significantly by lot differences.
Preventative Maintenance
Even the best chromatographer is going to face issues with their HPLC system and separations from time to time. It is important to use good practices when running the system to prevent or minimize instrument downtime. A structured preventative maintenance program will reduce unplanned breakdowns and should be carried out at least once a year. It is also recommended to run a system suitability test (and ideally, a column or method suitability test) before a batch of samples to make sure the instrument is working properly. Along with the system suitability test, other tests can be performed on a regular basis to identify any possible instrument issues. Such tests would include a gradient performance test (tests mixing accuracy), a flow rate test, a retention reproducibility test, and a peak-area reproducibility test.
It is extremely important to keep a good repair/ maintenance log and even a log for each column. The value of historical records in establishing instrument failure patterns and preventative maintenance programs cannot be underestimated. Keeping an inventory of spare parts can also lead to quicker turnaround times when something needs to be changed or replaced. Remember to work systematically to change one thing at a time, and that the problem happens more than once so it’s not a one-time issue.
Conclusion
Whether you are an experienced chromatographer or a novice, it is important to know when your HPLC system is not working properly. Knowing which possible problems to expect and what to inspect first can save you a lot of instrument downtime. Good historical records will also improve your productivity when troubleshooting and get your instrument back in working order.



