AUTHOR: Stephanie Schuster, PhD, Senior Technical Support Scientist, Advanced Materials Technology 20 Years of High…

Time and Solvent Savings by Modernizing USP Methods with HALO® Columns Following the New <621> Guidelines
Author: Dr. Stephanie Schuster, Senior Technical Support Scientist from Advanced Materials Technology
For more information, please contact: [email protected]
INTRODUCTION
The guidelines in United States Pharmacopeia (USP) Chapter <621> were recently updated to allow changes to particle size and column dimensions for gradient methods with verification instead of revalidation. This enables laboratories to benefit from modern column technologies which create both time and solvent savings. The advantages associated with moving from the use of traditional fully porous particles (FPPs) to innovative superficially porous particles (SPPs) stem from the SPPs providing faster, improved separations that consume less solvent. Conveniently, the switch to small SPPs does not impact the overall system suitability of the method.
CHANGES TO USP <621>
Although permissible changes for USP isocratic methods were enacted in 2014, modifications for gradient methods without revalidation went into effect on December 1, 2022. Similarly, British Pharmacopoeia (BP), European Pharmacopoeia (EP), and Japanese Pharmacopoeia (JP) changes were enacted in January 2023. Many of the <621> allowable gradient method parameter changes are the same as those for isocratic methods, such as stationary phase, column dimensions, internal diameter (ID), flow rate, pH, buffer, and injection volume. A different column must be in the same L category, but switching from a totally porous particle (TPP) to a superficially porous particle is permitted. Particle size and/or length of the column may be modified, provided that the ratio of the column length (L) to the particle size (dp) remains constant or in the range between −25% to +50% of the prescribed L/dp ratio. When changing from TPP to SPP in isocratic methods, other combinations of L and dp can be used, provided that the plate number (N) is within −25% to +50%, relative to the prescribed column. For changes from TPP to SPP in gradient methods, other combinations of L and dp can be used provided that the square of the ratio of retention time to peak width at half height (tR/Wh)2 is within−25% to +50%, relative to the prescribed column for all the peaks that are used to determine the system suitability parameters. All of these changes are acceptable provided system suitability criteria are fulfilled, and selectivity and elution order of the specified impurities to be controlled are demonstrated to be equivalent.
Allowable changes to mobile phase composition and column temperature for gradient methods are outlined in Chapter <621> and differ from isocratic methods. For isocratic, the amount of the minor components in the mobile phase can be adjusted by ±30% relative. However, the change in any component cannot exceed ±10% absolute. Changing the mobile phase in gradient methods is more complex. Gradient time may be adjusted by the equation: tG2=tG1×(F1/F2)[(L2×dc22 )/(L1×dc12)] in which tG1 is the initial gradient time, tG2 is the modified gradient time, F1 is the original flow rate, F2 is the modified flow rate. L1 and dc1 are the original column length and diameter, respectively, while L2 and dc2 represent the new column length and diameter. Column temperature can be changed ± 10 °C in isocratic methods, whereas a change of only ± 5 °C is permitted in gradient methods. Note that changes in detector wavelength are not acceptable for either type of separation.
While method changes may be overwhelming, there are two free online method translators to help. They can be found here and here.
OPTIMIZING THE MOVE TO SMALLER PARTICLE SIZES AND COLUMN DIAMETERS
It should be noted that smaller particle sizes and column IDs are more susceptible to extra-column band broadening. Also known as dispersion, sources of this band broadening in the LC system outside of the analytical column include the sample injector and pre-column tubing, as well as the post-column tubing and the flow cell/ionization source. In isocratic methods, the pre-column dispersion has the most impact. In contrast, post-column band broadening is most impactful in gradient methods.
There are several actions that can help reduce extra-column band broadening. For example, using the shortest and smallest ID tubing between instrument components with finger-tight zero dead volume connectors is a good start. The use of a smaller volume detector flow cell (≤ 2.5 µL) and increased data acquisition rate (≥ 20 Hz) are also recommended. Finally, decreasing the injection volume helps, but is more influential for isocratic methods than gradient separations. The effect of minimizing dispersion is shown in Figure 1, in which the extra-column volume has been reduced by a factor of four. As a result, a 50% average increase in plates is observed, as well as baseline separation of the highlighted peaks. Reducing the band broadening, therefore, helps maximize the performance of smaller particle sizes and smaller diameter columns.
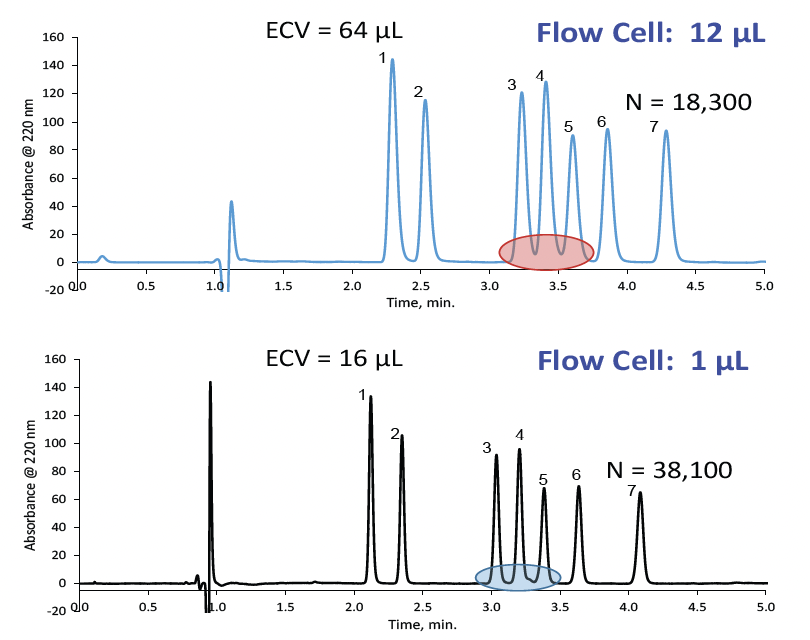
Figure 1: Extra-Column band broadening impact on resolution and efficiency with a HALO® 2 µm column.
PEAK IDENTITIES
- Cannabidivarinic acid (CBDVA)
- Cannabidvarin (CBDV)
- Cannabidiolic acid (CBDA)
- Cannabigerolic acid (CBGA)
- Cannabigerol (CBG)
- Cannabidiol (CBD)
- Tetrahydrocannabivarin (THCV)
TEST CONDITIONS
Column: HALO 90 Å C18, 2.0 µm, 2.1 x 150 mm
Mobile Phase A: water/0.1% Formic Acid
Mobile Phase B: ACN/0.1% Formic Acid
Isocratic: 75 %B
Flow Rate: 0.3 mL/min
Temperature: 30 °C
Detection: UV 220 nm, PDA
Injection Volume: 0.6 µL
Sample Solvent: 75/25 methanol/water
Data Rate: 100 Hz (Nexera) or 25 Hz (Prominence)
Response Time: 0.025 sec.
Flow Cell: as indicated
LC System: Shimadzu Nexera X2 (1 µL flow cell) or Shimadzu Prominence UFLC XR (12 µL flow cell)
SPP PARTICLES VS FPP PARTICLES
A traditional fully porous particle, also known as a totally porous particle, is porous throughout its entire body. The morphology of a superficially porous particle, however, is very different; it comprises a solid silica core surrounded by a porous silica shell. This structure enables high efficiency and high-speed separations. Figure 2 demonstrates the power of SPPs in a van Deemter plot of three FPPs compared to one SPP (Fused-Core®). In the graph of plate height versus linear velocity, lower plate heights equate to higher efficiency. As FPP particle size is decreased, the efficiency increases. However, the 2.7 µm SPP is more efficient than the 1.8 µm FPP even though it is larger. This is due to the coefficients in the van Deemter equation, shown in the figure, being smaller with SPPs. The Eddy diffusion term (A) is 30 – 40% lower, due to more uniform flow paths through the column bed compared to columns with FPPs. In addition, the longitudinal diffusion term (B) is 25 – 30% smaller for SPPs because of the solid core inside the particles. These lower coefficients result in lower plate height equivalents (H) and thus greater efficiency.
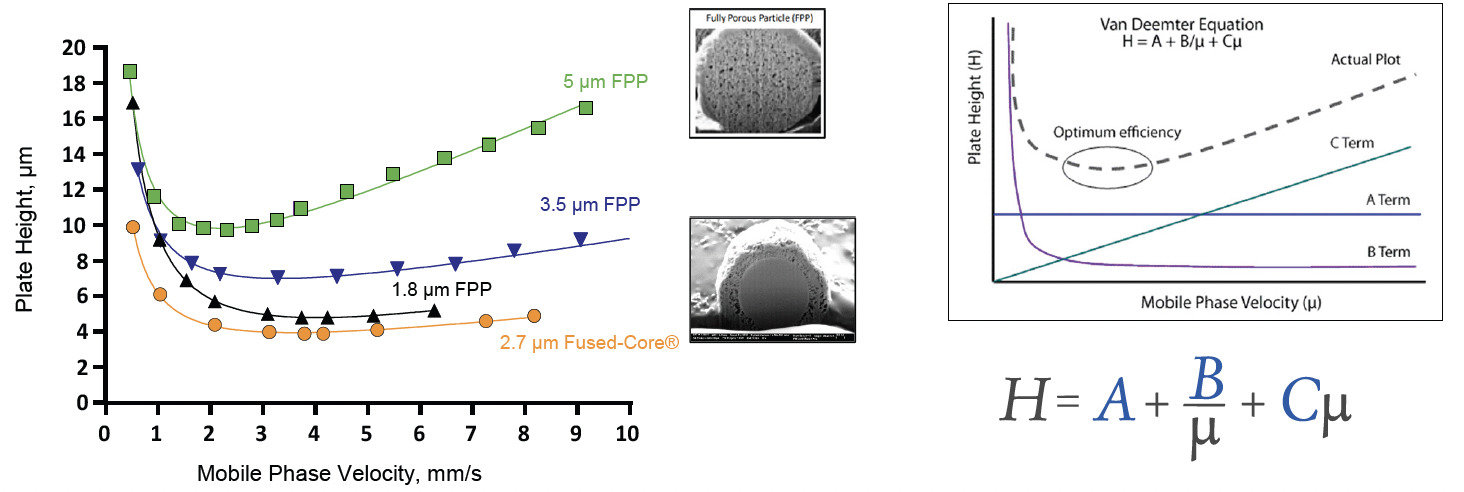
Figure 2: van Deemter comparisons of HALO® Fused-Core® to other FPP columns of different sizes showing the advantages of the SPP design.
van Deemter Equation
H = height equivalent to a theoretical plate
A = eddy diffusion term (particle size and how well bed was packed) 30 – 40% smaller
B = longitudinal diffusion term 25 – 30% smaller
C = resistance to mass transfer term (kinetics of the analyte b/w mobile phase and stationary phase)
µ = mobile phase linear velocity (L/t0)
Effect of Particle Size and Type
Columns: 4.6 x 50 mm, 5 µm FPP C18
3.5 µm FPP C18
1.8 µm FPP C18
2.7 µm HALO® C18
Solute: naphthalene
Mobile phase: 60% ACN/40% water
Temperature: 24 °C
The distinct advantage of SPPs over FPPs is demonstrated in Figure 3. The isocratic separation of fat-soluble vitamins using the traditional particles is shown in the bottom trace, which features unresolved peaks with low efficiency. In contrast, high-resolution peaks with baseline separation are observed in the top chromatogram, which utilized high-efficiency HALO® SPPs. In this example, only the particle morphology was changed; all other parameters were the same.
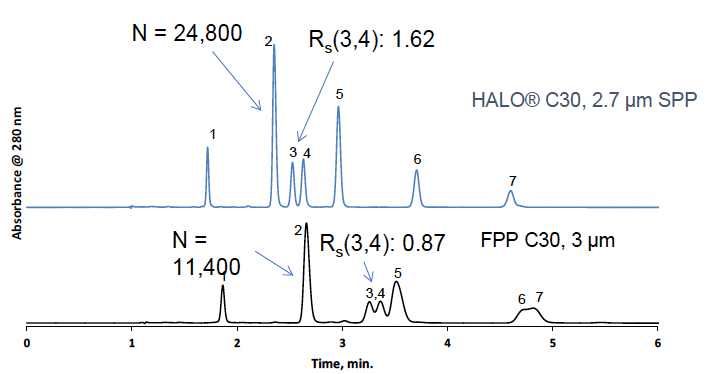
Figure 3: Increased efficiency and resolution demonstrated using fat-soluble vitamins analyzed using a HALO® C30 column.
PEAK IDENTITIES
- Retinyl acetate (A)
- Delta tocopherol (E)
- Ergocalciferol (D2)
- Cholecalciferol (D3)
- Alpha tocopherol (E)
- DL-alpha-tocopherol acetate (E)
- 2,3-trans-phylloquinone (K)
TEST CONDITIONS
Column: Morphology and phase as indicated, 4.6 x 150 mm
Isocratic: 100% Methanol
Flow Rate: 1.5 mL/min
Temperature: 30 °C
Detection: UV 280 nm, PDA
Injection Volume: 2 µL
Sample Solvent: methanol
Data Rate: 40 Hz
Response Time: 0.025 sec.
Flow Cell: 1.0 µL
LC System: Shimadzu Nexera X2
The higher efficiency of SPPs allows for the use of shorter columns without sacrificing resolution. In Figure 4, the top chromatogram shows the gradient separation of acids in a 4.6 x 250 mm column with 5 µm FPP Polar Embedded Amide particles. With a flow rate of 1.5 mL/min, the run time was 15 minutes. The bottom trace shows the results when a 2.1 x 100 mm column with 2 µm HALO® RP-Amide SPP particles was utilized. The separation was complete in just 3.75 minutes, a 4-fold improvement while achieving a better resolution between the peaks. Moreover, the smaller column and faster separation, combined with the reduction of flow rate to 0.5 mL/min, consumed 12 times less solvent.
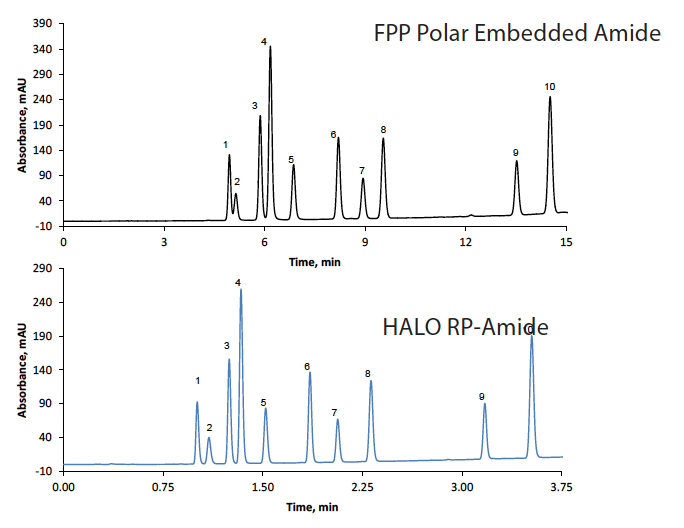
Figure 4: Increased speed and reduced mobile phase consumption by moving the method to a smaller particle size, smaller ID, and shorter length HALO® column. The back pressure generated would require a UHPLC instrument.
PEAK IDENTITIES
- homovanillic acid
- caffeic acid
- syringic acid
- vanillic acid
- chlorogenic acid
- sinapic acid
- ferulic acid
- p-coumaric acid
- trans-cinnamic acid
- resveratrol
TEST CONDITIONS
Columns:
FPP: 4.6 x 250 mm, 5 µm 1.5 mL/min, 30-60 %B in 15 min
HALO®RP-Amide: 2.1 x 100 mm, 2 µm 0.5 mL/min, 30-60 %B in 3.75 min
Mobile Phase A: 20mM Phosphoric Acid
Mobile Phase B: Methanol
Gradient: as indicated
Flow Rate: as indicated
Initial Pressure: 716 bar (HALO® column) 295 bar (FPP column)
Temperature: 35 °C
Detection: UV 220 nm, PDA
Injection Volume: 0.5 µL
Data Rate: 40 Hz
Response Time: 0.025 sec
Flow Cell: 1.0 µL
LC System: Shimadzu Nexera X2
CASE STUDIES: ISOCRATIC
Transitioning methods to SPPs allows improvements in resolution and faster run times. The chromatograms in Figure 5 show the dramatic effects of changing particle sizes and morphologies. The top trace shows the results of a separation using a 4.6 x 150 mm C18 column with 5 µm FPPs. Switching to an SPP particle, with all other parameters unchanged, shortened the run time and improved both the resolution and number of theoretical plates (N), as shown in the second chromatogram. According to the third trace, using a smaller SPP (2.7 µm) improved the resolution by almost 2.5x while shortening the run time even more. Finally, the run time was reduced by a factor of 3 by employing a 2.1 x 50 mm C18 column with even smaller (2 µm) SPPs, as depicted in the bottom chromatogram. Despite the significantly faster run time, the resolution and number of plates were still much higher than the original FPP run. Note that all SPP separations were permissible using the <621> L/dp guidelines.
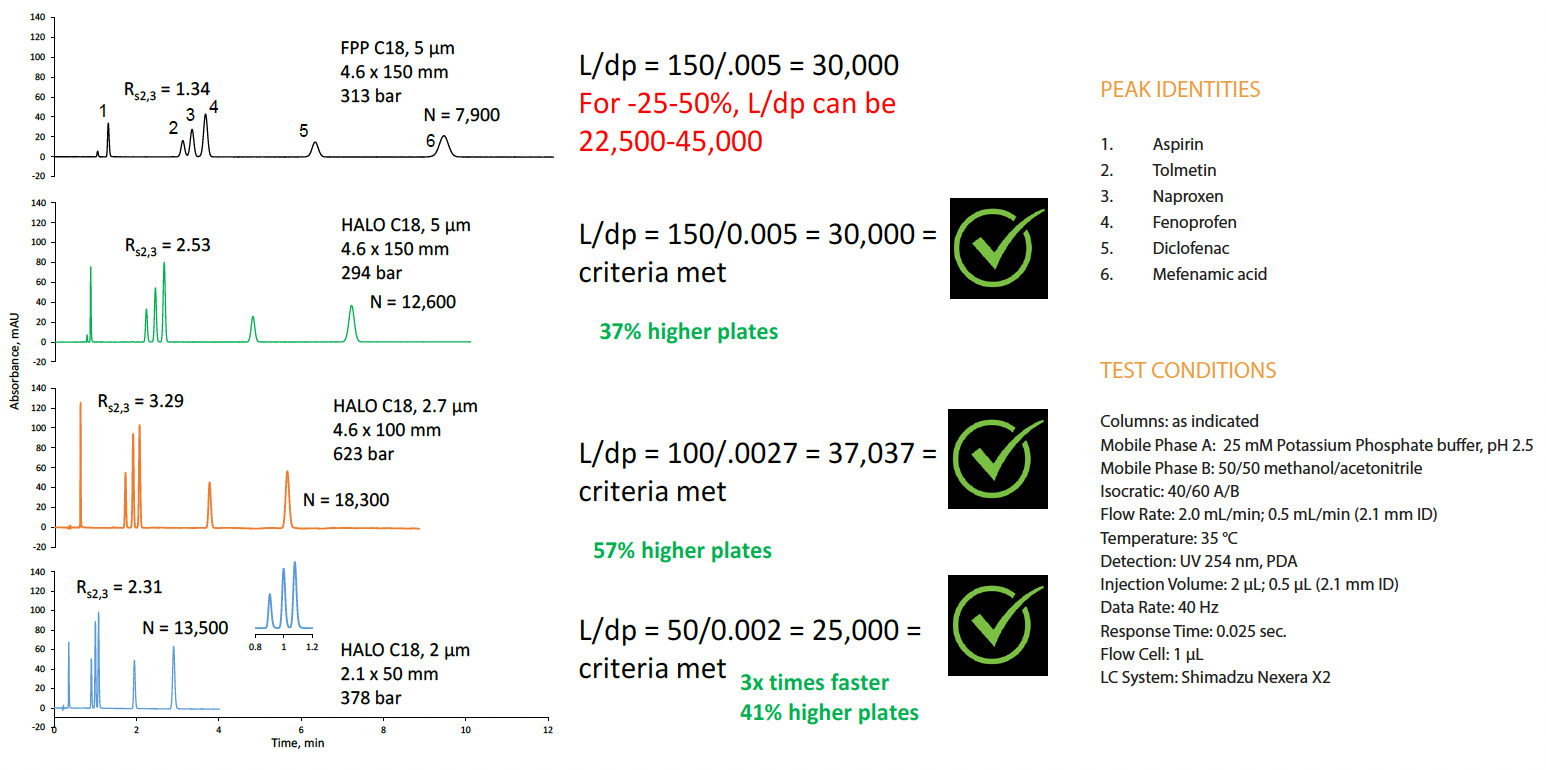
Figure 5. Examples of possible changes using the L/dp ratio for an isocratic method.
Figure 6 shows an example of the L/dp criteria not being met, but the change to a different column was allowed because the plate number (N) was within −25% to +50% of the original method. The bottom chromatogram demonstrates that by transitioning the method to use a shorter column and smaller particle with SPP morphology, in addition to minimizing the extra-column volume, the run time decreased by a factor of 6 with comparable resolution. Moving to smaller column dimensions and particles also achieved considerable solvent savings.
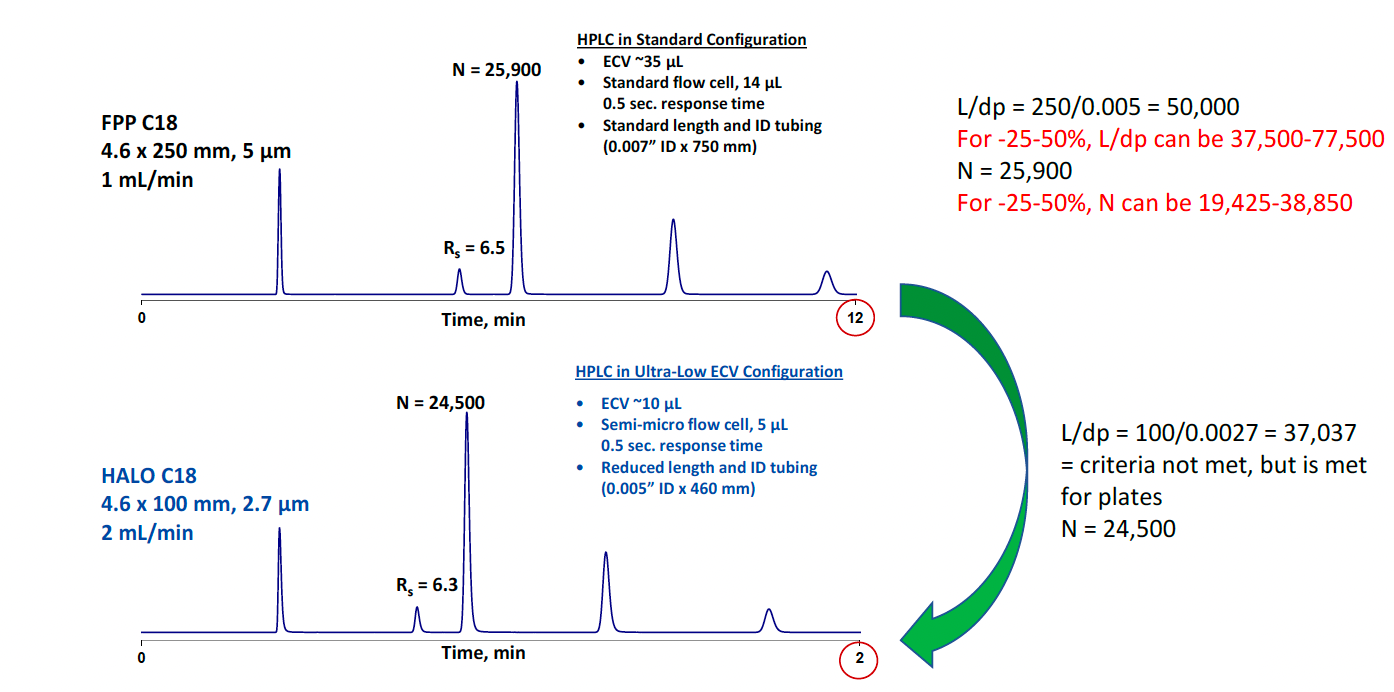
Figure 6: Example of the advantage of HALO® Fused-Core® columns: increased speed without loss of resolution using a shorter length column and reduced extra column volume.
PEAK IDENTITIES
- Uracil
- Benzonitrile
- Nitrobenzene
- 1-Chloro-4-nitrobenzene
- Toluene
TEST CONDITIONS
Columns: as indicated
Mobile Phase A: water
Mobile Phase B: acetonitrile
Isocratic: 50/50 A/B
Flow Rate: as indicated
Temperature: 30 °C
Detection: UV 254 nm
LC System: Agilent 1100
CASE STUDIES: GRADIENT
As stated in Chapter <621>, “Adjustment of conditions with gradient elution HPLC is more critical than with isocratic HPLC elution, since it may shift some peaks to a different step of the gradient, potentially causing partial or complete coelution of adjacent peaks or peak inversion, and, thus leading to the incorrect assignment of peaks and to the masking of peaks or a shift such that elution occurs beyond the prescribed elution time.” In other words, care must be taken to avoid changing the elution order of the peaks. For some parameters, the adjustments are explicitly defined in the monograph to ensure the system’s suitability.
Based on the updated <621> criteria, method optimization was explored for cobamamide, also known as adenosylcobalamin, which first entailed verification of the system suitability. The original USP monograph calls for using an SPP C18, 2.6 µm, 4.6 x 100 mm column with an injection volume of 5 µL and flow rate of 1.2 mL/min at 40 °C. The total run time is 45 minutes. Substituting a HALO® 90 Å C18 column with the same dimensions, but with the HALO® Fused-Core® 2.7 µm SPP particles easily meets the requirements for L/dp, as the new dimensions are so similar. All other parameters were unchanged. System suitability requires column efficiency to be no less than 22,000 plates and a tailing factor of no more than 2.0; the HALO® column easily passed both criteria while lowering the back pressure. In addition, the elution order of a system suitability mix remained unchanged, as shown in Figure 7.
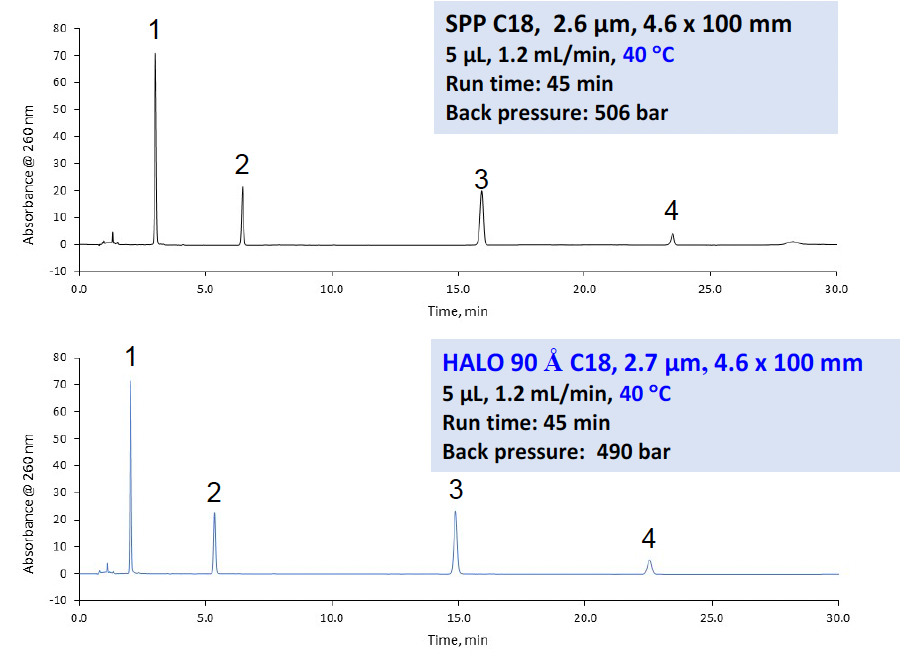
Figure 7: Original monograph column for cobamamide compared to the equivalent HALO® column showing that system suitability criterion is met for the HALO® column with no changes in elution order. The main difference is the particle size, but both are SPP columns.
PEAK IDENTITIES
- hydroxocobalamin chloride
- cyanocobalamin
- cobamamide
- methylcobalamin
TEST CONDITIONS
Columns: as indicated
Mobile Phase A: Potassium phosphate buffer, pH 3.2
Mobile Phase B: methanol
Gradient: as indicated in the monograph
Flow Rate: as indicated
Temperature: 40 °C
Detection: UV 260 nm, PDA
Injection Volume: 5 µL
Data Rate: 40 Hz
Response Time: 0.025 sec.
Flow Cell: 1 µL
LC System: Shimadzu Nexera X2
The particle distribution was analyzed for both the original monograph column and the HALO® column as given in Figure 8. Comparing the results showed that there was no significant difference in particle size and there is a slightly better standard deviation for the HALO® particles.
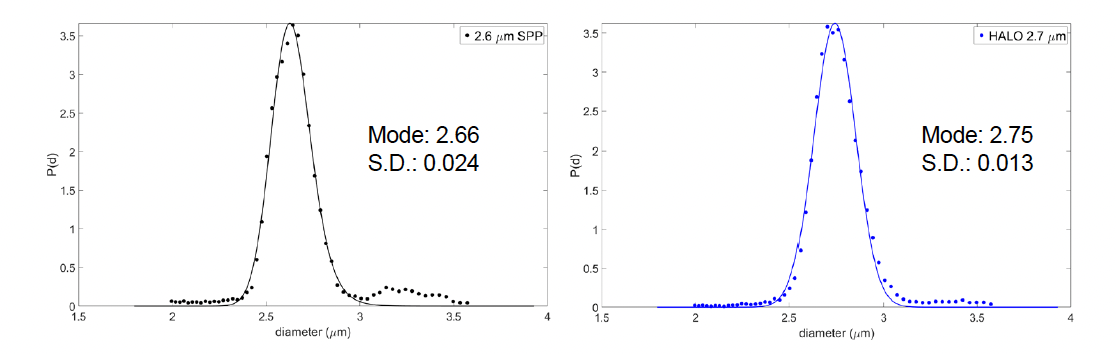
Figure 8. Comparison of particle size distributions.
To speed up the separation, the column was exchanged for a HALO 90 Å C18, 2 µm, 2.1 x 75 mm column. The flow rate was increased to 0.4 mL/min which is slightly higher than the same linear velocity as the original method and the injection volume was reduced to 0.8 µL by this equation: Vinj2=Vinj1 × (L2d2c2 )/(L1d2c1 ). Allowing for equilibration, the total run time was reduced from 45 minutes to 25.5 minutes. Following this modification, N and tailing factor were suitable. Figure 9 shows that the modified method meets the USP requirements for L/dp while shortening the run time by a factor of 1.8. In addition, the smaller column allowed less solvent to be used. The initial monograph consumes 54 mL/run whereas the modified method needs only 10 mL/run, leading to considerable savings.
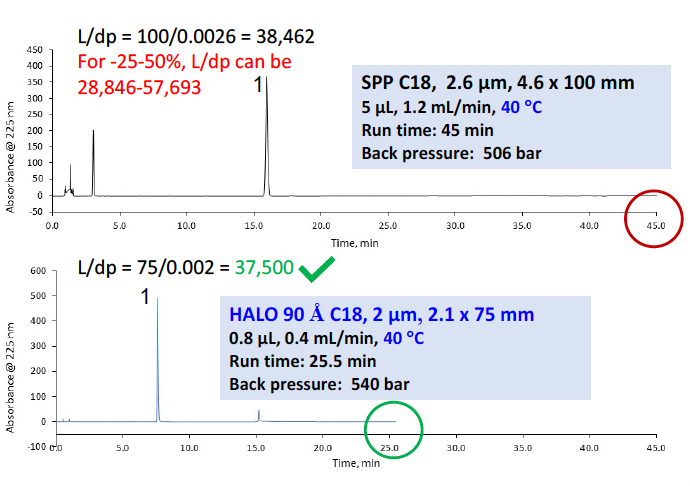
Figure 9: Original monograph compared to the modernized method for cobamamide demonstrating 1.8 times the speed and 5.4 times the solvent savings while still meeting the system suitability criteria.
PEAK IDENTITIES
- cobamamide
TEST CONDITIONS
Columns: as indicated
Mobile Phase A: Potassium phosphate buffer, pH 3.2
Mobile Phase B: methanol
Gradient: as indicated in the monograph
Flow Rate: as indicated
Temperature: 40 °C
Detection: UV 260 nm, PDA
Injection Volume: 5 µL
Data Rate: 40 Hz
Response Time: 0.025 sec.
Flow Cell: 1 µL
LC System: Shimadzu Nexera X2
Similar steps were taken to modernize the method for itraconazole, an antifungal medication. The monograph calls for a C18, 3 µm, 4.6 x 100 mm FPP column, 10 µL injection, 1.5 mL/min flow rate, 30 °C, with a gradient of 20-50 %B in 12 minutes and a total run time of 20 minutes. Keeping everything the same except for the substitution of a HALO 90 Å C18 column with 2.7 µm SPPs, system suitability was easily passed. Then, employing a smaller HALO 90 Å C18, 2 µm, 2.1 x 50 mm column and adjusting the injection volume and flow rate accordingly, substantially shortened the run time while still meeting the USP requirements. As depicted in Figure 10, the total run time was three times faster with the HALO® column while ten times less solvent was used. This example demonstrates the significant advantages of the HALO® Fused-Core® SPP particles.
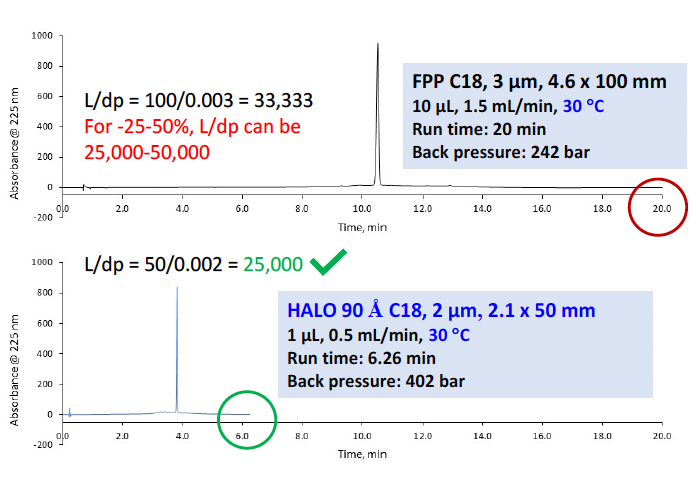
Figure 10: Original monograph compared to the modernized method for itraconazole demonstrating more than 3 times the speed and 10 times the solvent savings while still meeting the system suitability criteria.
TEST CONDITIONS
Columns: as indicated
Mobile Phase A: 13.6 g/L of tetrabutylammonium hydrogen sulfate
Mobile Phase B: ACN
Gradient: Time %B
4.6 x 100 mm 0.00 20
12.00 50
16.00 20
20.00 20
2.1 x 50 mm Time %B
0.00 20
3.75 50
5.00 20
6.25 20
Flow Rate: as indicated
Pressure: as indicated
Temperature: 30 °C
Detection: UV 225 nm, PDA
Injection Volume: as indicated
Sample Solvent: 0.4% HCl in methanol
Data Rate: 100 Hz
Response Time: 0.025 sec.
LC System: Shimadzu Nexera X2
The monograph method for rivaroxaban, a prescription blood thinner, specifies a C18, 3.5 µm, 3.0 x 150 mm FPP column, 3 µL injection, 1 mL/min flow rate, 60 °C, with an initial hold at 2 %B for 2 min and then a segmented gradient of 2-16 %B in 6 minutes, 16-36 %B in 17 minutes, 36-80 %B in 12 minutes and a total run time of 45 minutes. The method was modernized by moving to a smaller particle size, shorter length HALO® column as shown in Figure 11. The flow rate was reduced to 0.5 mL/ min and the gradient was scaled for a total run time of 27 minutes. The injection volume was reduced to 0.9 µL. The system suitability criteria were met for the HALO® column with resolution between peaks 3 and 4 NLT 8.0. Tailing and %RSD criteria were almost met when the HALO® column was used. By modernizing the method, it was sped up by 1.7 times and used 3.3 times less mobile phase compared to the original monograph method. The back pressure is slightly higher, but well within the operating parameters of an HPLC system, which means that a UHPLC is not required.
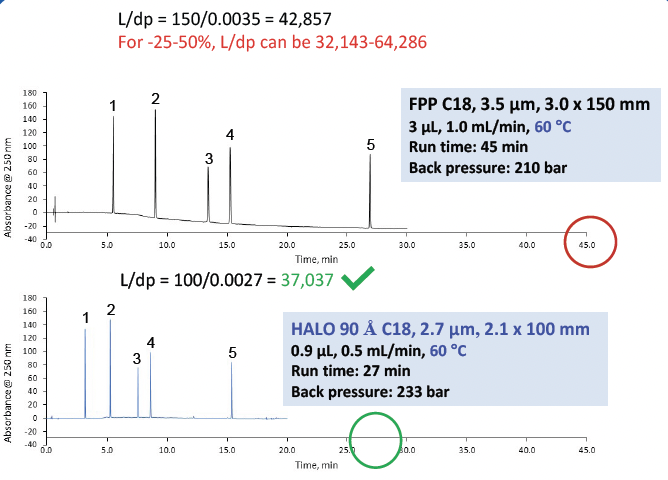
Figure 11. Original monograph compared to the modernized method for the organic impurities of rivaroxaban demonstrating time and solvent savings while still meeting the system suitability criteria.
PEAK IDENTITIES
- Rivaroxaban related compound B
- Rivaroxaban related compound D
- Rivaroxaban related compound G
- Rivaroxaban
- Rivaroxaban related compound J
TEST CONDITIONS
Column: FPP C18, 3.5 µm, 3.0 x 150 mm
Column: HALO 90 Å C18, 2.7µm, 2.1 x 100 mm
Part Number: 92812-602
Mobile Phase A: 5/95 Methanol/Solution A
Mobile Phase B: Acetonitrile
Flow Rate: as indicated
Pressure: as indicated
Temperature: 60 ⁰C
Detection: UV 250 nm, PDA
Injection Volume: as indicated
Sample Solvent: 40/60 Acetonitrile/Solution B
Data Rate: 40 Hz
Response Time: 0.025 sec.
Flow Cell: 1 µL
Instrument: Shimadzu Nexera X2
Gradient: Time %B
FPP C18, 3.5 µm, 3.0 x 150 mm
2.00 2
8.00 16
25.00 36
37.00 80
38.00 2
45.00 2
HALO 90 Å C18, 2.7 µm, 2.1 x 100 mm
Time %B
1.14 2
4.56 16
14.26 36
21.10 80
22.00 2
27.00 2
Summary
Superficially porous particles deliver striking improvements to chromatographic separations compared to fully porous particles. HALO® SPP columns in particular are made for ruggedness with high efficiency and high-speed separations. However, it is important to optimize the instrumentation in order to gain the most benefit from this technology for both isocratic and gradient methods. Following new USP guidelines for gradient method modernization enables both FPP and SPP methods to be improved for speed and solvent savings using HALO® Fused-Core® column technology while avoiding revalidation.



