AUTHOR: Conner McHale, Technical Support Specialist, Advanced Materials Technology INTRODUCTION to PFAS ANALYTICAL METHODS Per-…

Water and Fat-Soluble Vitamin Separations Using the HALO® C18 and AQ-C18 Phases
AUTHOR:
Peter Pellegrinelli, M.S., Applications Specialist
ABSTRACT
Vitamins are a crucial nutrient to humans and animals alike. In order for people to live healthy lives they need the right nutrients to enable these healthy conditions. Today, people are more conscious of their health and can often choose to supplement their daily vitamin intake with many different over the counter products. While some people choose these products to supplement their vitamin intake, some must use these supplements in order to be healthy usually due to some sort of disorder or metabolic syndrome. To monitor these disorders or deficiencies, periodic testing must be conducted by a clinical laboratory. These clinical samples can be very low in concentration which require sensitive LCMS systems and high efficiency separations for adequate detection and quantitation. Most vitamins are of interest when testing clinical samples and these vitamins can be either water- or fat-soluble. Choosing an appropriate column chemistry and optimizing the LCMS method conditions coupled with a new 1.5 mm ID column by HALO® increases sensitivity through signal intensity thereby allowing for improved detection and quantitation.
INTRODUCTION
Vitamin deficiencies can come in multiple forms. Some medical illnesses can make it difficult to properly absorb the necessary vitamins. These illnesses can be anything from kidney disease, Crohn’s disease, or even liver disease among others not listed. To confirm that someone has one of these deficiencies, a blood sample is taken to test the vitamin levels in their system. This is where HPLC and LCMS are employed. The samples analyzed on an HPLC or LCMS system must have adequate resolution of the various vitamins as well as any vitamers or metabolites. These water- and fat-soluble vitamins can be difficult to separate without a robust method and column. HALO 90 Å C18 is recommended for fat soluble vitamins while HALO 90 Å AQ-C18 is recommended for water soluble vitamins.
Elevated levels or vitamin deficiencies are determined by testing blood samples for the metabolites. These metabolites are direct products from the metabolism of the vitamin precursors. On the following page are two separations of vitamin D/B6 and their respective metabolites/vitamers. Both of these vitamins are important to monitor either for bone density (vitamin D) or a weakened immune system (vitamin B6). Each separation was performed on the phases mentioned above with simple mobile phases. For the vitamin D metabolites, a column ID comparison was performed to provide an example of increased sensitivity when moving from larger column IDs to smaller IDs. This is important for patient testing to accurately determine the concentration of metabolites in a patient sample. It is not uncommon that the concentration of the compound of interest is very low with clinical samples. With larger ID columns this low concentration sample may be hard to quantify. By switching to a smaller ID column there is an increase in sensitivity that can make it easier to quantify these compounds of interest, provided that the same sample concentration and volume are injected on the smaller ID column. Using the new HALO® 1.5 mm ID column these separations are possible to be done in a quick and reproducible manner.
KEY WORDS:
Metabolites, Vitamin D2, Vitamin D3, Vitamin B6, HALO® C18, Pyridoxamine, Pyridoxal, Pyridoxal phosphate, Pyridoxine, 25-hydroxy-D2, 25-hydroxy-D3, clinical testing, 1.5 mm, semi- micro
EXPERIMENTAL DATA: Method Conditions: Vitamin D2/D3 Metabolites
Column: HALO 90 Å C18, 2.7µm, 2.1×100 mm
Part Number: 92812-602
Column: HALO 90 Å C18, 2.7µm, 1.5×100 mm
Part Number: 9281X-602
Mobile Phase
A: Water, 0.1 % Formic Acid
B: ACN, 0.1 % Formic Acid
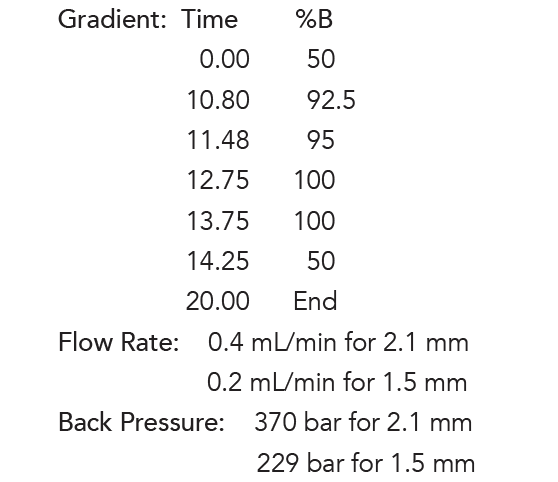
Flow Rate:
0.4 mL/min for 2.1 mm
0.2 mL/min for 1.5 mm
Back Pressure:
370 bar for 2.1 mm
229 bar for 1.5 mm
Temperature: 40 °C
Detection: +ESI/APCI MS/MS Injection
Volume: 1.0 µL @ 500 ppb
Sample Solvent: 50/50/ ACN/H₂O MS
Source Conditions: ESI +/APCI
Spray Voltage: 4.5 kV
Nebulizing gas: 2 L/min
Drying gas: 15 L/min
DL temp: 250 ˚C
Heat Block: 300 ˚C
RESULTS
VITAMIN D2/D3 METABOLITES:
Vitamin D metabolites 25-Hydroxyvitamin D2 and D3 are separated on HALO® C18 with a comparison between column ID sizes. The metabolites have no UV absorption which means they must either be run with LC-MS detection or ELSD detection. A sensitivity comparison was run for the metabolites by switching to the smaller 1.5 mm ID column. The 1.5 mm ID column gave an increase in intensity and area counts over the 2.1 mm ID along with an increase in signal to noise.
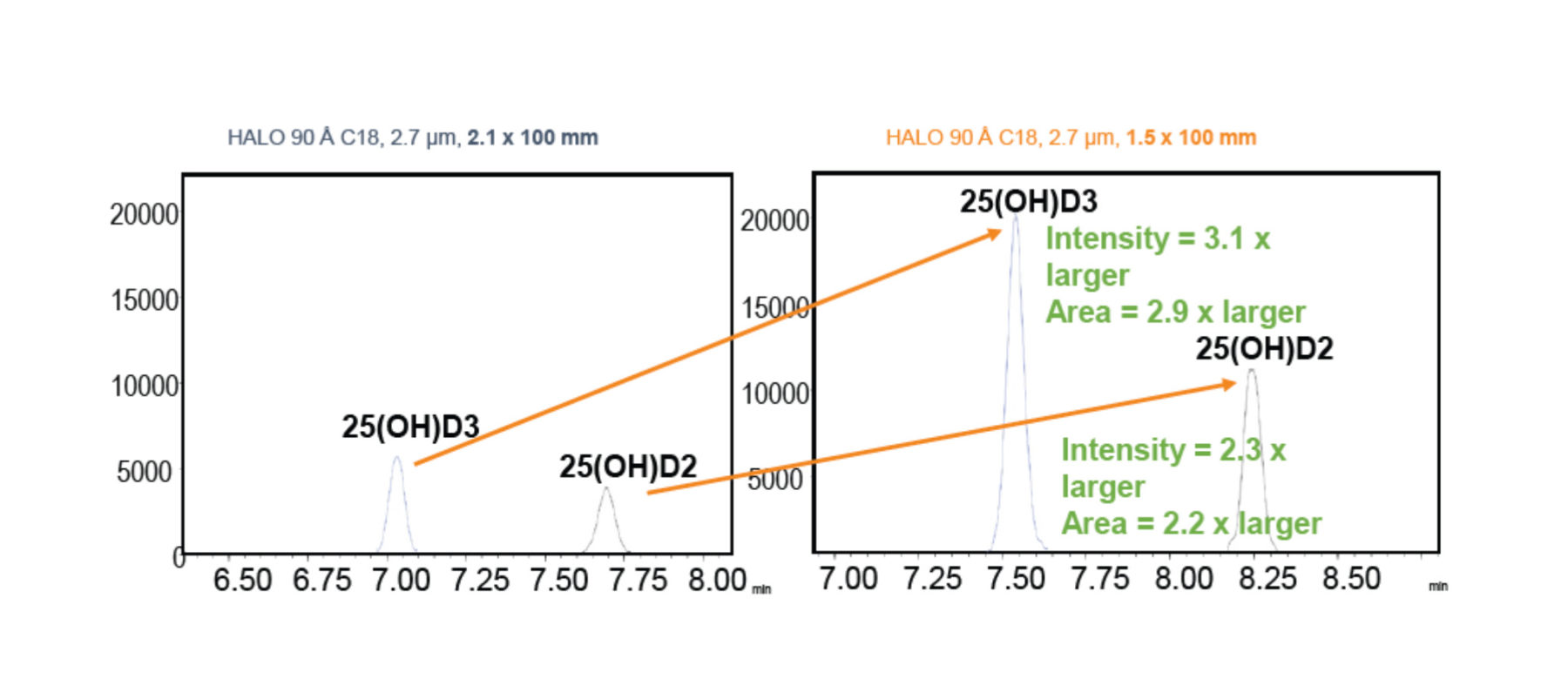
Figure 1. Gradient separation of vitamin D metabolites on a 2.1 and 1.5 mm ID column. Peak Identities are metabolite D3 (25-hydroxyvitamin D3) and metabolite D2 (25-hydroxyvitamin D2).
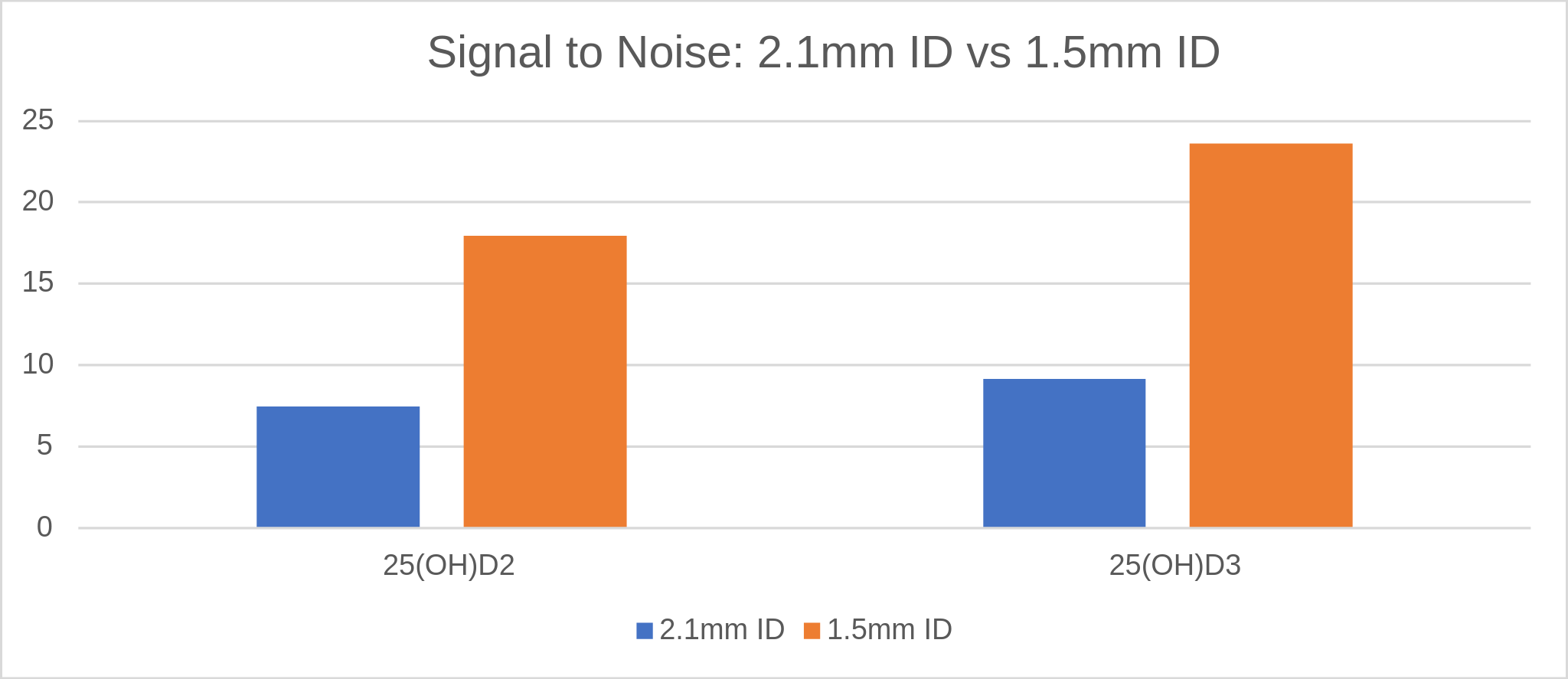
Graph 1. A signal to noise comparison for both vitamin D metabolites showing the difference from the 2.1mm ID to the 1.5 mm ID.
|
Peak |
Compound |
Retention Time |
Target Mass |
|
|
2.1 mm ID |
1 |
25-Hydroxy D3 |
7.018 |
395.3 |
|
2 |
25-Hydroxy D2 |
7.675 |
383.4 |
|
|
1.5 mm ID |
1 |
25-Hydroxy D3 |
7.528 |
395.3 |
|
2 |
25-Hydroxy D2 |
8.237 |
383.4 |
|
Table 1. A table detailing the retention times and MS parameters for the vitamin D metabolite separations.
The above mass spectra show the separation of both metabolites D2 and D3 on two different columns. The metabolites have less retention on the 2.1 mm ID column compared to the 1.5 mm ID. A major difference in the chromatography is the sensitivity of the 1.5 mm ID column. The 1.5 has almost a three-fold increase in area counts for D3 and over three-fold increase in intensity over the signal to noise 2.1 column. The bar graph above also demonstrates that with the increase in sensitivity for the 1.5 ID that signal to noise also increases. This helps verify that noise does not also increase with the smaller ID column. Both columns show excellent resolution between both metabolites with a simple gradient.
Method Conditions: Vitamin B6 Vitamers
Column: HALO 90 Å AQ-C18, 2.7µm, 1.5 x 150 mm
Part Number: 9281X-722
Mobile Phase
A: 5mM Ammonium Formate, 0.1 % Formic Acid
B: MeOH, 0.1 % Formic Acid
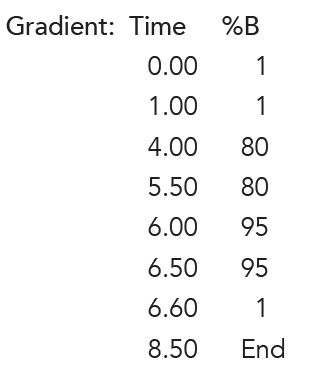
Flow Rate: 0.2 mL/min
Back Pressure: 395 bar
Temperature: 30 °C
Detection: +/-ESI MS
Injection Volume: 1.0 µL
Sample Solvent: Water
MS Source Conditions: ESI +/-
Spray Voltage: 4.5 kV
Nebulizing gas: 2 L/min
Drying gas: 15 L/min
DL temp: 250 ˚C
Heat Block: 300 ˚C
RESULTS
VITAMIN B6 VITAMERS:
B6 vitamers are separated on the HALO® AQ-C18 phase. The vitamers do not absorb in the UV spectrum so the separation must be run by MS detection. All four of the samples were run in positive mode.
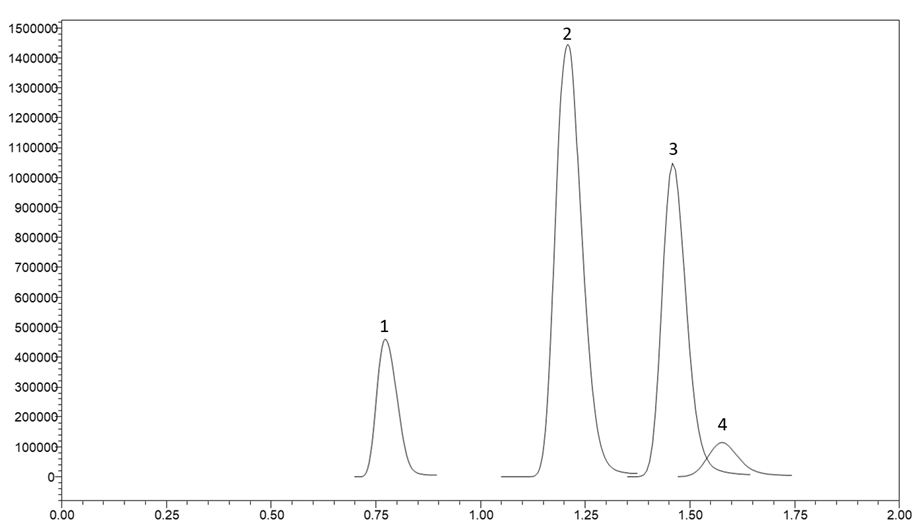
Figure 2. A gradient separation of four vitamin B6 metabolites on a HALO® AQ-C18 column. Peak identities in order are Pyridoxamine, Pyridoxal, Pyridoxine, and Pyridoxal phosphate.
|
Peak |
Compound |
Retention Time (Minutes) |
Target Mass (m/z) |
Transition (m/z) |
|
1 |
Pyridoxamine |
0.78 |
169.1 |
134.1 |
|
152.1 |
||||
|
2 |
Pyridoxal |
1.23 |
168.0 |
149.9 |
|
3 |
Pyridoxine |
1.48 |
170.0 |
151.9 |
|
4 |
Pyridoxal Phosphate |
1.56 |
248.0 |
150.1 |
|
94.1 |
||||
|
133.0 |
Table 2. A table detailing the retention times and MS parameters for the B6 vitamer separations.
The vitamin B6 vitamers were all able to elute from the column quickly with good peak shape. With less than a two minute separation, the compounds were well separated and would allow high throughput for a laboratory running hundreds of samples per day.
CONCLUSION:
Vitamins play an important role in human metabolism. People with various disorders or deficiencies need to constantly be tested to maintain healthy amounts of vitamins. With the need for testing, diagnostic facilities must use robust and reproducible methods with sensitive detection to accurately determine patient vitamin levels. With the examples shown above, the new HALO® 1.5 mm ID columns can not only produce well resolved chromatograms, but can also increase the sensitivity of these separations over common 2.1 mm ID columns. The HALO® 1.5 mm ID columns are easy to implement into commercial UHPLC and LCMS systems and also provide an added benefit of using 50% less solvent consumption in comparison to the 2.1 mm ID columns.
REFERENCES:
- https://www.fda.gov/consumers/consumer-updates/fda-101-dietary-supplements (June 2, 2022)
-
Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014 Mar 20;21(3):319-29. doi: 10.1016/j. chembiol.2013.12.016. Epub 2014 Feb 13. PMID: 24529992; PMCID: PMC3968073.
-
Parra M, Stahl S, Hellmann H. Vitamin B₆ and Its Role in Cell Metabolism and Physiology. Cells. 2018 Jul 22;7(7):84. doi: 10.3390/cells7070084. PMID: 30037155; PMCID: PMC6071262.
-
Jiang, X., Wang, Y., & Liu, J. (2023). Comprehensive characterization of amino acids and water‐soluble vitamins in a pentylenetetrazole‐induced seizures rat model. Journal of Separation Science, 2201004.



