AUTHOR: Stephanie Schuster, PhD, Senior Technical Support Scientist, Advanced Materials Technology 20 Years of High…

A Novel Screening Approach for Comparing LC-MS Reversed-Phase and HILIC Methods for Separations in Biological Matrices Using Amino Acid Examples
AUTHOR:
Conner McHale, Technical Support Specialist and Taylor Harmon, Ph.D., R&D Scientist
ABSTRACT
Metabolomic analyses are utilized in various avenues of clinical research, including drug discovery, disease characterization, and pharmacodynamic evaluation. Metabolites are often studied via liquid chromatography-mass spectrometry (LC-MS) in both reversed-phase (RPLC) and hydrophilic interaction (HILIC) modes. Yeast extract and heat-deactivated human serum were utilized as model biological systems to demonstrate the orthogonal selectivity achievable with the use of both HILIC and RPLC separations in the analysis of amino acids. A novel gradient technique was employed to save time and to avoid the need to prepare new mobile phases when RP columns are exchanged for HILIC columns. Generating LC-MS comparisons with RP or HILIC columns can be done with the same mobile phase reservoirs while changing only gradient profiles to suit RP or HILIC mode. This screening technique can be easily automated and expanded by using column switching valves.
INTRODUCTION
The characterization of small molecule metabolites in biofluids such as urine, plasma, and bile are important in drug discovery and disease characterization. Metabolomics assays have been successful in the discovery of novel molecular pathogenetic mechanisms and have seen utility in drug development and pharmacokinetic characterization [1]. Whether targeted analysis (a specific panel of compounds) or untargeted (measuring as many metabolites as possible) mass spectrometry combined with high-performance liquid chromatography (LC-MS) is the ideal choice for detection/ quantification.
One challenge in metabolomics is retention of polar analytes using reversed-phase liquid chromatography (RPLC) using low or no organic in the mobile phase. Although initial experiments might give adequate results, stationary phase dewetting causes drastic reduction in column performance upon repeat separations, including retention loss. HALO® C18 columns are one of the most popular hydrophobic phases in the world but, due to possible dewetting, should not be used with mobile phases that contain less than about 10% organic solvent. HALO® AQ-C18 phase has been developed by Advanced Materials Technology with a polar embedded group in the stationary phase, which resists dewetting effects. Operating in a totally aqueous environment can offer many separation advantages, particularly with highly polar compounds. When compared to standard hydrophobic C18 phases, the HALO® AQ-C18 can be used with a broader range of mobile phase polarities and demonstrates greater retention and reproducibility under high aqueous conditions. HALO® AQ-C18 also shows different retention and selectivity for certain polar compounds which make it an ideal complement to HALO® C18 when slightly different selectivity is needed. An example of this behavior can be seen in Figure 1 on the next page, which demonstrates a separation of nucleobases under 100% aqueous conditions.
KEY WORDS:
metabolites, metabolomics, amino acids, superficially porous particles, HPLC, HILIC
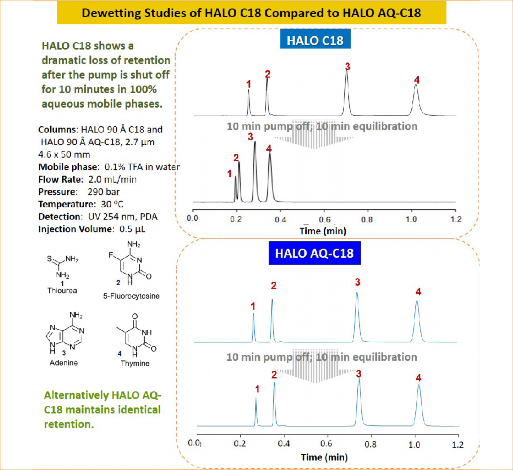
Figure 1: Dewetting studies of HALO® C18 Compared to HALO® AQ-C18
Figure 1 shows a mixture of nucleobases separated with 100% aqueous mobile phases. Once the separation is completed, the pump is stopped for 10 minutes followed by a 10 minute equilibration with injections repeated. The C18 column shows a drastic loss in retention due to phase dewetting, whereas the AQ-C18 maintains retention due to its polar embedded group within the stationary phase.
One alternative to RPLC for very polar compounds is HILIC. The HALO® Penta-HILIC (available in 2, 2.7, and 5 µm) is a proprietary penta-hydroxy containing ligand (See Figure 2) that provides a highly polar bonded stationary phase compatible with all typical HILIC operating conditions. The fast column equilibration, due to the Fused-Core® particle technology, is especially useful for gradient separations. HALO® Penta-HILIC is particularly well suited for the separation of highly polar compounds that have low retention or poor solubility under highly aqueous reversed-phase conditions. Another advantage often reported for HILIC methods is an improvement in MS signal intensity due to the high organic- content mobile phases that enable more efficient electrospray droplet desolvation [2].
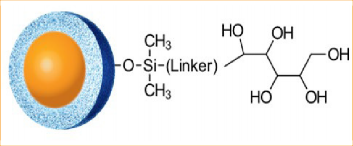
Figure 2: HALO® Penta-HILIC stationary phase structure
EXPERIMENTAL:
All experiments were run on a Shimadzu Nexera UHPLC instrument (Columbia, MD) coupled to a Thermo Q-Exactive HF Hybrid mass spectrometer (Bremen, Germany). Yeast extract, heat-deactivated human serum, LC-MS grade methanol, ammonium acetate, and ammonium formate were purchased from Sigma Aldrich (St. Louis, MO), LC-MS grade water and LC-MS grade acetonitrile from GFS Chemicals, and LC-MS grade formic acid from Thermo Scientific.
RPLC separations were performed on a HALO 90 Å AQ-C18, 2.7 µm, 2.1 x 150 mm column. HILIC separations were run on a HALO 90 Å Penta-HILIC, 2.7 µm, 2.1 x 150 mm column. Both columns were procured from Advanced Materials Technology, Inc. (Wilmington, DE).
Yeast extract stock was prepared at 5 mg/mL in 9:1 5 mM ammonium acetate:methanol. This stock was diluted 1:5 in water and acetonitrile for RPLC and HILIC, respectively, for a final concentration of 1 mg/mL. Solution was injected without further adulteration.
For RPLC-HRMS analysis, serum (100 µL) was mixed with 800 µL of 8:1:1 acetonitrile:methanol:acetone with 1 % (v/v) formic acid to precipitate proteins, which were pelleted via centrifugation (10,000 rcf for 10 min at 4°C). Supernatant was dried down by vacuum concentrator (35°C, 2 hrs.) and resuspended in 100 µL 5 mM ammonium acetate. Solution was again centrifuged per above parameters, and supernatant (80 µL) was removed for injection. For HILIC-HRMS analysis, serum (100 µL) was mixed with 500 µL of 9:1 acetonitrile: acetone with 0.1 % (v/v) formic acid to precipitate proteins, which were pelleted via centrifugation (10,000 rcf for 10 min at 4 °C). Supernatant was removed and injected directly onto the instrument.
HALO® AQ-C18 TEST CONDITIONS:
Column: HALO 90 Å AQ-C18, 2.7 µm 2.1 x 150 mm
Part Number: 92812-722
Mobile Phase A: 8 mM ammonium formate, pH 3.0 (aq.), in 100 % water
Mobile Phase B: 8 mM ammonium formate, pH 3.0 (aq.), in 95:5 acetonitrile:water

Flow Rate: 0.5 mL/min
Temperature: 35°C
Detection: LC/MS QExactive HF Hybrid Orbitrap
Injection Volume: 2 µL
HALO® Penta-HILIC TEST CONDITIONS:
Column: HALO 90 Å Penta-HILIC, 2.7 µm 2.1 x 150 mm
Part Number: 92812-705
Mobile Phase A: 8 mM ammonium formate, pH 3.0 (aq.), in 100 % water
Mobile Phase B: 8 mM ammonium formate, pH 3.0 (aq.), in 95:5 acetonitrile:water
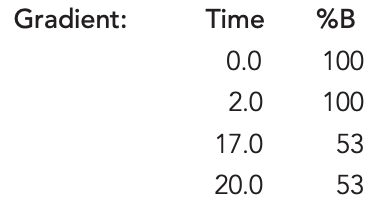
Flow Rate: 0.3 mL/min
Temperature: 35°C
Detection: LC/MS QExactive HF Hybrid Orbitrap
Injection Volume: 2 µL
RESULTS:
Amino acid metabolism is involved in myriad metabolic mechanisms, including tissue growth, energy production, immune function, and nutrient absorption. Isomers such as leucine and isoleucine can be challenging to separate via RPLC since they are often completely unretained under even slightly organic elution conditions. Figure 3A shows the reversed phase separation results on the AQ-C18 column which provide sufficient retention for the isomer separation of leucine and isoleucine during the isocratic hold of the separation.
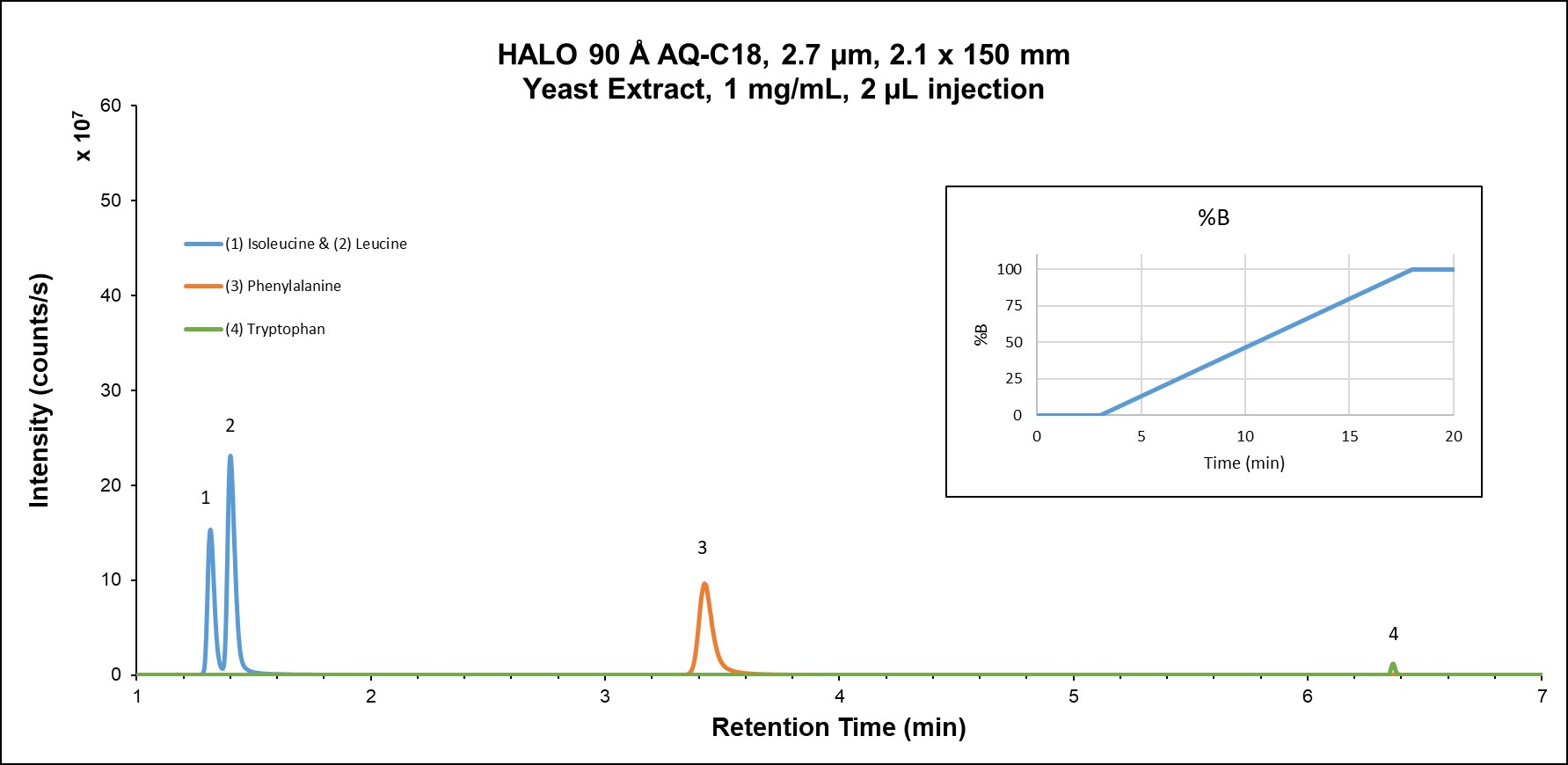
Figure 3A: Amino acids in yeast extract on HALO® AQ-C18 using reversed phase
RPLC exhibits higher selectivity than HILIC in this example; however, very polar metabolites are not well retained by RPLC, so HILIC is often necessary for complete separation of all compounds. HILIC mode provides more retention for all analytes, as well as altered selectivity. Figure 3B demonstrates the high degree of resolution (including isomers) in addition to the orthogonal selectivity that can be attained in HILIC mode. It is also important to note the higher sensitivity that HILIC mode gives due to the higher percentage of organic solvent in the mobile phase.
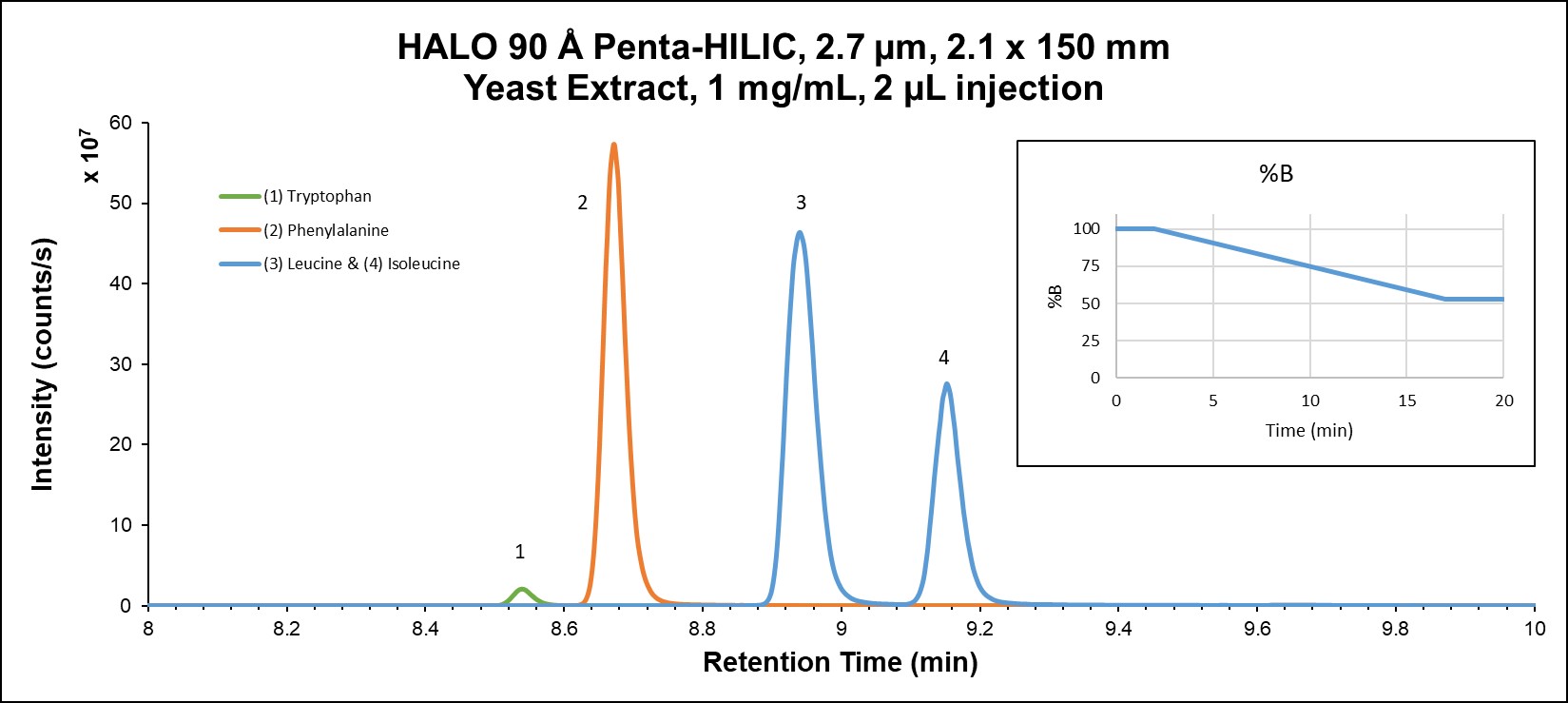
Figure 3B: Amino acids in yeast extract on HALO® Penta-HILIC using HILIC mode
Just as highly polar compounds elute in the void volume in RPLC, highly non-polar metabolites elute in the void volume in HILIC, and ion suppression can therefore cause the number of fat-soluble features to decrease substantially. In this perspective, RPLC and HILIC methods are complementary with respect to metabolite coverage and best used in parallel to accomplish global analysis. [3] It is important to ensure that different sample matrices show similar results and repeatability. Both the HALO® AQ- C18 and Penta-HILIC stationary phases exhibit excellent inter-matrix compatibility, as demonstrated in the analysis of yeast extract and heat-deactivated human serum in Figure 4 below.
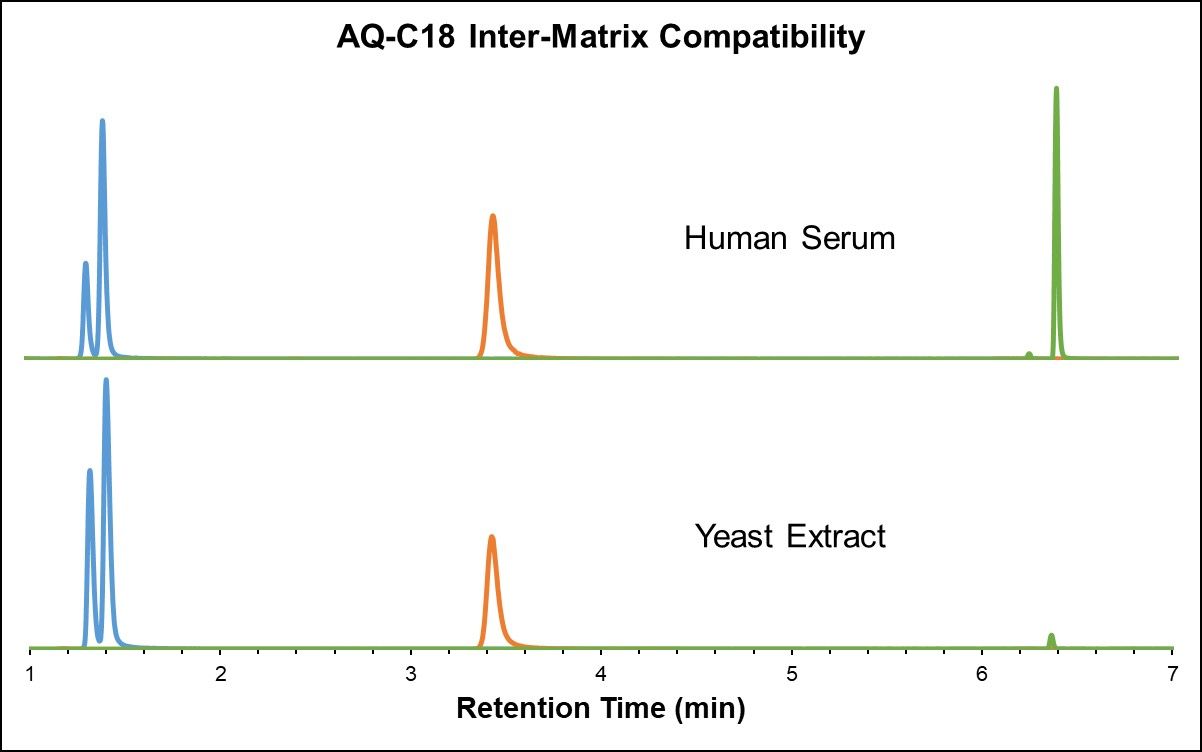
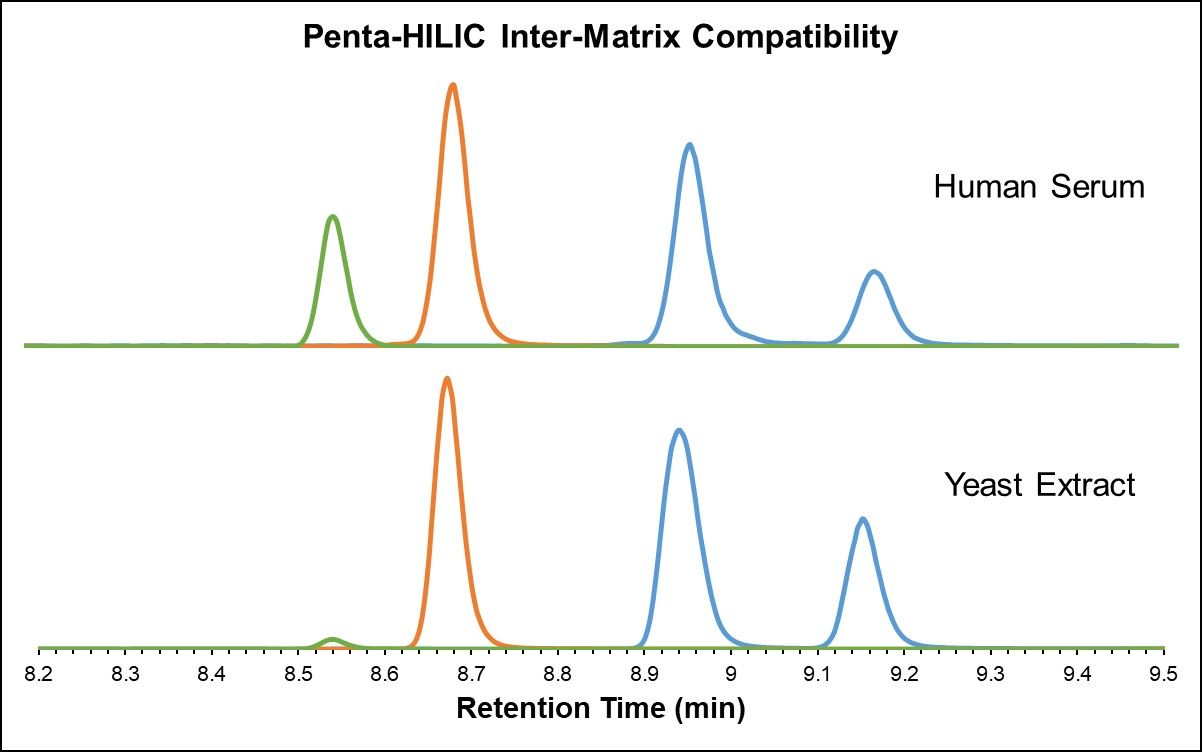
Figure 4: Amino acids in yeast extract and heat-deactivated human serum (AQ-C18 and Penta-HILIC)
CONCLUSION:
LC-MS is a commonly used analytical technique in the pharmaceutical industry because of its versatility for performing qualitative and quantitative analysis. Separation conditions, including the mode of chromatography utilized, play a significant role in method development for achieving resolution requirements. Polar metabolites can pose challenges for an RP separations. Retention of polar analytes often requires a highly aqueous mobile phase that may be problematic for typical RP workflows and column technology, and decreased ionization efficiency may be observed in electrospray ionization mass spectrometry (ESI-MS) under high aqueous conditions [4]. Fully dewettable columns need to be considered when working with polar analytes for adequate robustness, resolution, and retention. Chromatographers should investigate benefits of transferring methods to HILIC mode when retention of analytes is too low and resolution or sensitivity is not achieved with RP separations. HALO® Penta-HILIC columns are useful tools for the analysis of polar metabolites and can be utilized to achieve higher sensitivity and better resolution than comparable RP separations.
A novel method has been demonstrated for screening RP and HILIC modes by preparing A and B mobile phases and allowing the instrument to reverse the gradient profiles to compare column retention by both nonpolar (RP) and polar (HILIC) mechanisms. The system may be easily automated to save additional time in screening columns if a column selection valve is included.
REFERENCES
- Iwasaki Y, Sawada T, Hatayama K, Ohyagi A, Tsukuda Y, Namekawa K, Ito R, Saito K, Nakazawa H. Separation technique for the determination of highly polar metabolites in biological samples. Metabolites. 2012 Aug 16;2(3):496-515. doi: 10.3390/metabo2030496. PMID: 24957644; PMCID: PMC3901216.
- Ray, L.B., Science 2010, 330, 1337, Spagou, K., Tsoukali, H., Raikos, N., Gika, H., Wilson, I. D., Theodoridis, G., J. Sep. Sci. 2010, 33, 716-727.
- Patti, G. Separation strategies for untargeted metabolomics. J. Sep. Sci. 2011, 34, 3460-3469
- Iwasaki Y, Sawada T, Hatayama K, Ohyagi A, Tsukuda Y, Namekawa K, Ito R, Saito K, Nakazawa H. Separation technique for the determination of highly polar metabolites in biological samples. Metabolites. 2012 Aug 16;2(3):496-515. doi: 10.3390/metabo2030496. PMID: 24957644; PMCID: PMC3901216.



